
From Malaria (Ed. I. Sherman) ASM Press, Wash. D.C. 1998
Invasion - Merozoites
Merozoites are small ovoid cells 1.5-2.5µm in length and 1.0-2.0µm in width .


From Malaria (Ed. I. Sherman) ASM Press, Wash.
D.C. 1998
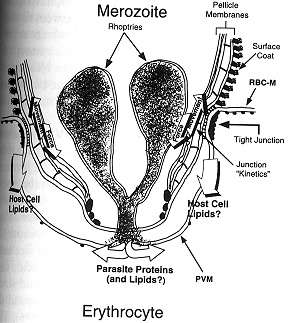
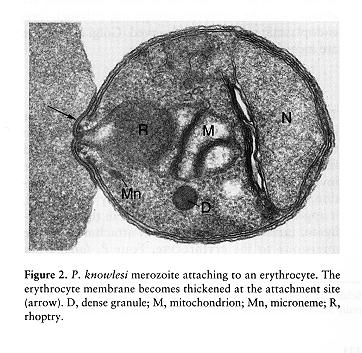
They have a surface coat, pellicular membrane complex, nucleus, chromosomes, mitochondria, endoplasmic reticulum, a Golgi apparatus, cytostome, microspheres, polar rings, microtubules and several apical organelles that are specialized for invasion of erythrocytes: rhoptries and micronemes. The initial interaction between the merozoite and red cell surface is by random collision and involves reversible binding, however in order for the merozoite to invade it must re-orient itself so the apical end is in contact with red blood cell surface. The attachment and reorientation of the merozoite is via the interaction of the merozoite surface protein, MSP-1, and its proteolytically processed products (as well as perhaps several other MSPs) with specific red blood cell receptors. Micronemes contain a variety of proteins that serve to irreversibly bind the merozoite to specific receptors on the red cell surface. In P. vivax and P. knowlesi discharge of a 135-140kD protein binds to the Duffy factor, whereas in P. falciparum the protein has a molecular size of 175kD (and is called the erythrocyte binding antigen, EBA-175) and binds to glycophorin A. The genes encoding these micronemal erythrocyte- binding proteins have been cloned and sequenced.
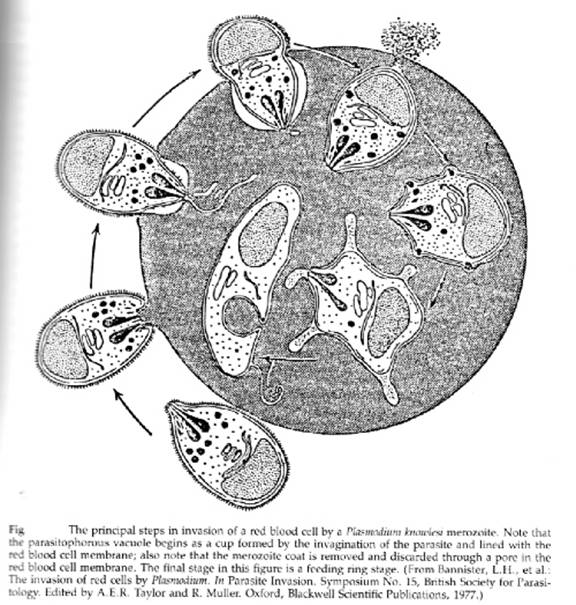
The rhoptries are a pair of club shaped organelles that contain the apical membrane antigen 1 (AMA-1) which is presumed to bind to red cells because antibodies to it block invasion, high molecular weight Rhop proteins and low molecular weight RAP proteins, and several proteases. After discharge of micronemes and rhoptries a tight junction forms, the merozoite glides forward by means of its actin-myosin motor, and the red cell membrane invaginates to enclose the merozoite within a parasitophorous vacuolar membrane (PVM). The entire process takes less than a minute. The critical role of the actin-myosin motor in entry has been demonstrated by the use of cytochalasin B: drug-treated merozoites attach but do not enter. It may be that the rhoptry constituents bind to the red cell or the merozoite to promote adhesion or to facilitate expansion of the PVM.
From Malaria (Ed. I. Sherman) ASM Press, Wash.
D.C. 1998
When the merozoite has entered the red blood cell the microspheres move to the parasite’s surface, passing through the pellicular complex of membranes, releasing their contents by exocytosis into the vacuolar space, and this promotes the expansion of the PVM. Numerous microsphere proteins including RESA (ring erythrocyte surface antigen) and two subtilisin-like serine proteases have been isolated and localized to these 80nm spherical bodies.
The feeding and growing stage, the trophozoite
Once enclosed by the parasitophorous vacuolar membrane the merozoite de-differentiates, losing its apical organelles, and the pellicular complex. Devoid of the rigid pellicle it becomes the uninucleate ameboid feeding stage or trophozoite.
Some of the invaginations and extensions of the trophozoite surface can be quite large and after staining with a Romanowsky (such as Giemsa or Wright) stain and examination by light microscopy they resemble a signet ring, and therefore are referred to as ring forms. The trophozoite ingests host cell cytoplasm through a specialized circular feeding organelle, the cytostome. The host cell cytoplasm (principally hemoglobin) enters the cytostomal cavity; a bulge is pinched off opposite the cytostomal opening together with the cytostomal wall creating a food vacuole within the cytoplasm of the trophozoite. The acidic (pH 5.0-5.4) food vacuole contains at least three proteases, falcipain and plasmepsin I and II. Proteolysis of hemoglobin is an ordered process with plasmepsin I, an aspartic acid protease, cleaving native hemoglobin and plasmepsin II and falcipain, acidic cysteine proteases, cleaving denatured hemoglobin.
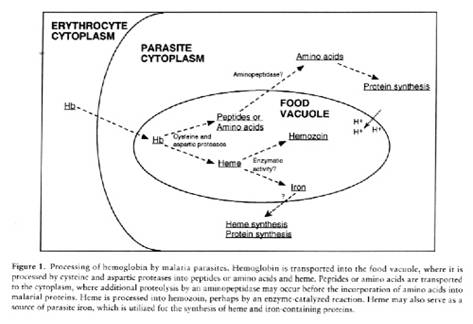
It is not clear whether the hemoglobin is fully degraded to amino acids or whether peptides derived from globin are transported from the food vacuole into the cytoplasm where they are further hydrolyzed. Inhibition of plasmepsin and falcipain activity results in parasite death. Work is in progress to crystallize these proteases and to use the derived structures to design novel antimalarials.
The amino acids required by Plasmodium for protein synthesis are provided by: hemoglobin, plasma amino acids and de novo synthesis. Globin serves as the principal source of amino acids for the growing plasmodium; since isoleucine, methionine and cysteine are deficient in mammalian globin these are obtained from the blood plasma. A limited number of amino acids may be formed by carbon dioxide fixation. Once the globin has been proteolyzed the free heme of hemoglobin in the food vacuole is polymerized into inert malaria pigment, hemozoin.
Release of heme results in its iron being oxidized to the ferric state, and the electrons liberated by this promote the formation of reactive oxygen intermediates such as superoxide radicals, hydroxide radicals, and hydrogen peroxide. These are detoxified by superoxide dismutatse and catalase, which are found in the food vacuole. The presence of polymerized particulate heme causes the food vacuole to be denser than other compartments of the cell. Consequently, the food vacuole can be isolated by density gradient centifugation thus enabling analysis of its contents.
Malaria parasites synthesize proteins in a typical eukaryotic manner; little is known about ribosome biogenesis but the ribosomes are 80S, with subunits of 60 and 40S, and have a low G+C content (32%) typical of protists. Malaria parasites are unusual in having very few ribosomal genes, and individual ribosomal genes are transcribed at different times by the various stages in the life cycle
Malaria parasites contain no carbohydrate reserves such as glycogen or amylopectin and therefore rely on continuous host-supplied glucose in the blood. A parasitized red cell may consume 75 times more glucose than an uninfected red cell. Plasmodium is a microaerophilic anaerobe incompletely degrading glucose to lactic acid via glycolysis.
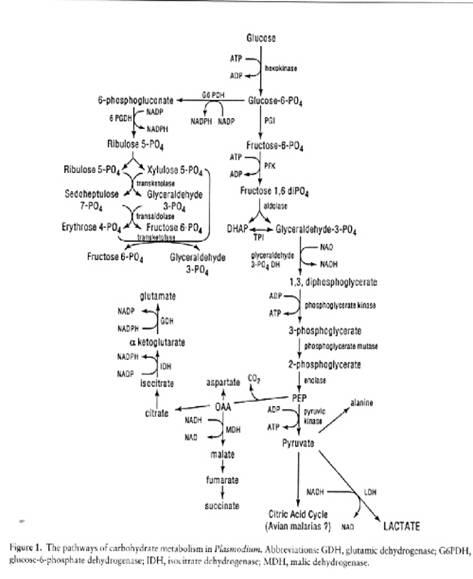
All of the glycolytic enzymes have been identified and some have been cloned and sequenced. The parasite also possesses a complete hexose monophosphate, and can fix carbon dioxide. The erythrocytic stages of human malarias lack cristate mitochondria and there is no evidence for a functional tricarboxylic acid cycle.
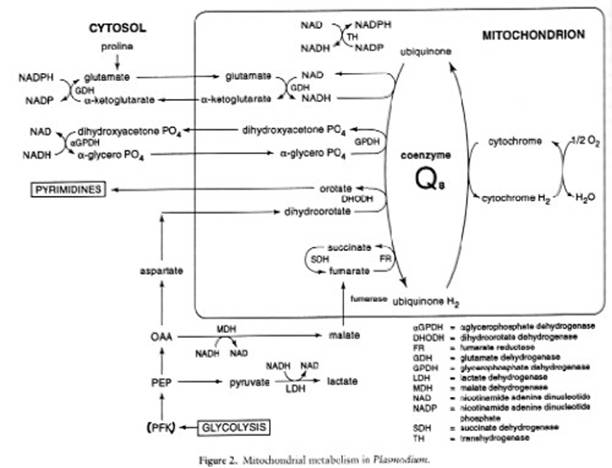
The Plasmodium-infected erythrocyte consists of a complex membrane system whose composition has not been thoroughly analyzed owing to the absence of efficient fractionation procedures. The parasite is unable to synthesize cholesterol or fatty acids de novo relying on dynamic exchanges with the host blood plasma for these as well as glycerol. The parasite, however, can chain elongate and saturate fatty acids and has the enzymatic machinery for synthesizing phospholipids from these building blocks. Drugs that specifically target plasmodial membrane biogenesis are under development.
The parasite lives within a "parasitophous vacuole" (PV) in the RBC that is formed during the invasion event. It makes and exports an elaborate membrane network that connects the PV with the outer membrane of the RBC. This network is called the tubovesicular membrane (TVM). Blocking the assembly of the network by inhibition of a parasite sphingomyelin synthetase by PPMP blocks delivery of exogenous nucleosides to the parasite (as well as other small molecules). This was examined by incubating cells with the flourescent dye, Lucifer yellow, which enters infected but not unifecte cells. In a normal infected cell, LY was detected in the tubules and vesciclesof the TVM and within the parasite. PPMP treated cells were blocked in the accumulation of LY within the red blood cell and parasite. PPMP also inhibited the parasite-induced uptake of labeled adenosine into the red cell. The uptakeof labeled glutamate was also inhibited.
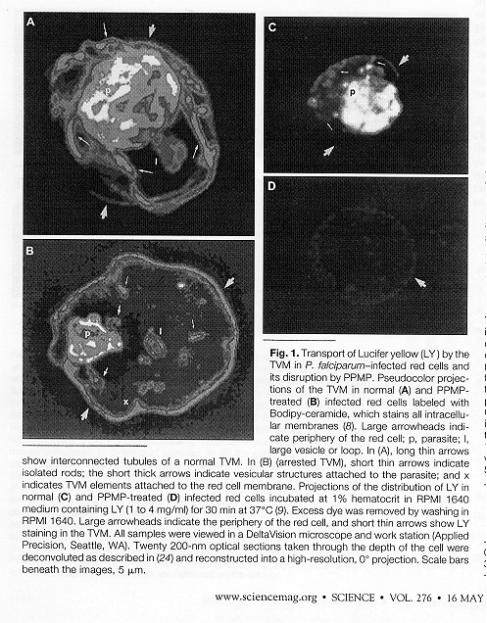
But, secretion of plasmodial proteins (such as the knob proteins) is not dependent on the TVM. The export of PfEMP2 and Mauer's clefts, knobs, etc. to the membrane and cytosol of theinfected cell was not affected. The manner by which the parasite secretes proteins into the PV, the RBC cytoplasm or the RBC plasma membrane is not known. TVM is a transport network that the parasite has evolved to get nutrients into the PV. TVM might also be exploited by scientists as a means to deliver antimalarial drugs directly to the parasite.
Caveolae-vesicle complex
When P. vivax-infected erythrocytes are stained with Giemsa stain and examined under the light microscope the surface has a punctate appearance. By electron microscopy these dots, called Schuffner’s dots, represent caveolae into which stain has been deposited.
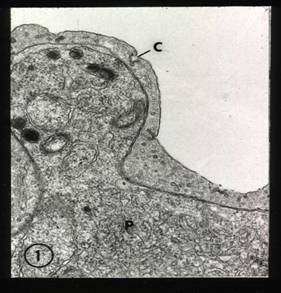

Caveolae-vesicle complex by TEM
Radiating from the caveolae (C) into the red cell cytoplasm are vesicles(right panel). The function of the caveolae-vesicle complex and their manner of formation are not known.
The Food/Digestive Vacuole
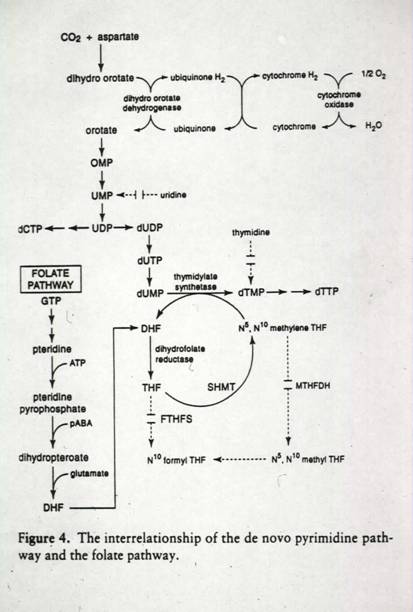
The parasite lives and divides inside the PV. Yet another vacuole, the food vacuole (also referred to the digestive vacuole) is formed within the parasite. This vacuole is an acidic organelle, pH5.0-5/4 and might be likened to a lysosome in a typical eukaryotic cell. Hemoglobin (Hb) comprises 95% of cytosolic protein of RBC. Host cytoplasm is consumed by growing trophozoite. Once the Hb is in the parasite, it is degraded in the food vacuole to get free amino acids needed for parasite growth. Plasmodium cannot make at least 5 amino acids and such must derive these from Hb. Hb degradation is essential for parasite survival – thus a good target for chemotheraphy. Hemozoin (polymerized heme) causes the food vacuole to be denser than other compartments in the cell. Thus the vacuole can be isolated, based on its density, by density gradient centrifugation. Characterization of purified food vacuoles has allowed the demonstration that Hb digestion is carried out by two classes of parasitic proteases: aspartic and cysteine proteases. Specific inhibitors of different classes of proteases were used to initially show this. Two essential aspartic proteases were purified: Plasmepsin I and II. One essential cysteine protease purified: Falcipain. Plasmepsin I is expressed in early rings and through-out trophozoite stage, Plasmepsin II is first expressed in trophozoites and falcipain is expressed in trophozoites and later stages. If you block plasmepsin I – parasite dies. Work is in progress to crystallize all these proteases and to use the structures derived from these studies to design new anti-malarial drugs.
Knobs are formations on the RBC membrane which are composed primarily of parasite proteins. The major knob protein is the "histidine-rich protein". Many other parasite proteins are also found in knobs, such as Plasmodium falciparum-infected erythrocyte membrane protein 1 (PfEMP1). Only infected RBCs have these knob-like structures. Proteineous, sticky knobs on RBCs that harbor growing trophozoites and schizonts cause these forms of the parasite to be sequestered in endothelia of vessels and thus to be removed from the peripheral blood. The only circulating infected RBCs are those harboring young trophozoites (rings) and gametocytes. Since the infection cycle in RBCs is synchonized, no parasites will be found in the peripheral blood during the latter part of the cycle (later growth stages and schizogony). Blood smears must be taken and examined periodically over a 24 hour period to assure detection of circulating, infected RBCs.
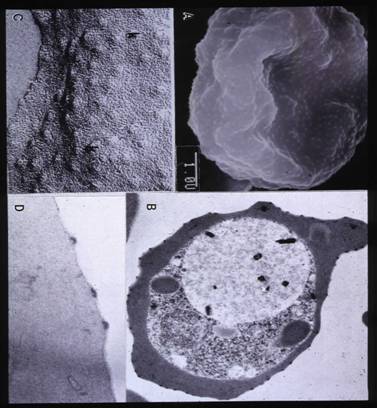
Knobs by A SEM, B TEM, C Freeze-fracture and D. TEM
Knobs are 40nm in height and 90-100nm in width, with an underlying electron-dense material, that contains several parasite proteins including histidine –rich protein. PfEMP 1 (Plasmodium falciparum erythrocyte membrane protein 1) as well as a modified form of the intrinsic red cell membrane protein band 3 are associated with the knob.
Maurer’s clefts
The parasite exports proteins such as the knob-associated histidine rich protein (KAHRP) by a novel pathway using parasite-derived membranous sacs in the red cell cytosol, Maurer’s clefts. To follow the trafficking pathway the coding sequences of the C-terminal of KAHRP linked to the green fluorescent protein (GFP) was used to generate a fusion protein in the transfection vector pHH2, and after transfection the infected red cells were observed for fluorescence. The first 60 amino acids of KAHRP were found to be sufficient for protein to move from the endoplasmic reticulum into the parasitophorous vacuole ((PV), but sequence elements with the additional 63 amino acids of KAHRP were necessary to move the protein from the PV into the red cell cytosol. Although it was believed that this pathway, unlike that using the TVM, was brefeldin B insensitive, experiments showed that although KAHRP transport was indeed brefeldin B sensitive it was necessary to add the drug when parasites were at the very early ring stage to block the pathway. In more mature stages KAHRP was transiently associated with Maurer’s clefts and FRAP (fluorescence recovery after photobleaching) analysis of the fluorescent signal for the cleft associated KAHRP-GFP showed quick recovery following photobleaching suggesting that the fusion protein in the red cell cytosol was in equilibrium with that in the clefts. Since PfEMP1 was co-localized with KAHRP in the clefts it appears that assembly of the knob structure takes place in Maurer’s clefts before its insertion into the red blood cell membrane.
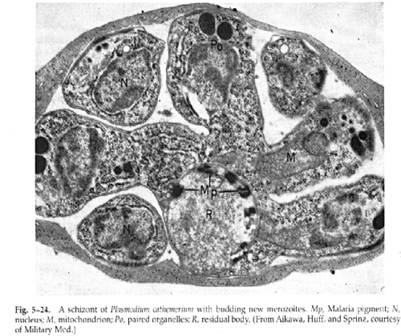
The dividing stage, the schizont or meront
This stage is recognized by its large size, the presence of larger amounts of hemozoin, and the presence of 8-24 nuclei. At the time of schizogony nuclear division and cytoplasmic organelle differentiation occur. The nuclear membrane remains intact during nuclear division and several nuclei lie free in the plasmodial cytoplasm; mitochondria divide and organelles that had disappeared during trophozoite development now reappear. Following the formation of the inner pellicular complex, rhoptry precursors form at the outer periphery, the various organelles migrate into regions enclosed by the inner membrane that protrude into the parasitophorous vacuolar space, and new merozoites are formed. Thus, in schizogony, unlike budding, nuclear division precedes cytoplasmic division. Egress of the merozoites occurs in about one second and appears to occur via a tube with a 2.5µm opening that is the result of fusion of the PVM with the red cell membrane. What remains after the merozoites leave the erythrocyte is a host cell membrane devoid of hemoglobin, the ghost, enclosing the PVM and the hemozoin-laden residual body.