Introduction to Protozoa and Parasites

All living organisms live in close interdependence on other living
organisms. One type of this interdependence is called symbiosis, which
means "living together".
The three categories of symbiosis are:
-
Commensualism is defined as "eating at the same table". The
association is not obligatory.
-
Mutalism involves a benefit to both organisms and the association
is obligatory.
-
Parasitism is the intimate association of two species where one
species (the parasite) benefits at the expense of the other (the host). The
parasite usually does not kill the host outright but profits by the advantages
of living on a surviving host. A dead host is usually not of use to the
parasite, although death of the intermediate host may in some cases provide a
means for reaching the definitive host.
These three categories are actually intersecting sets which blur at the
intersections.
Some definitions:
The "definitive" host is the host in which the parasite becomes
sexually mature.
The "reservoir" host is a non-human host that maintains
the infection in nature.
The "intermediate" host contains the larval or
asexual stages of the parasite.
Parasites that require a host for survival are
called "obligate" parasites.
"Facultative" parasites can survive
outside the host.
Endoparasites live inside a host and
ectoparasites live on the surface of the host.
Some generalizations about parasitism:
-
Parasites produce many progeny.
-
Parasites usually produce some impairment in the host, which can can be
subclinical or clinical. When the disease is severe, we call the organism pathogenic or
virulent
-
Parasites usually have resistant or specialized stages for moving between
hosts.
-
The life cycles are often complex, with the parasite living in several
hosts in different physiological stages.
-
Parasitism is widespread in both animals and protists. It probably evolved
independently multiple times.
-
Parasites are usually smaller than the host.
-
Many protozoal parasites are asexual.
-
Infectiousness is the ability of the parasite to gain entry and
maintain itself within the host.
History of Parasitology and Tropical Medicine
Helminth parasites were known in ancient Greece, Rome, China and the Arab
world. They were thought to arise by spontaneous generation. Malaria has been
recognized as a disease of man for over 4000 years. Reference to malaria can be
found in a Chinese document from 1700 BC. In ancient Greece, Hippocrates
classified different fevers as quotidan, tertian and quartan. He described
spleen enlargement and sickness from drinking water from marshy places.
Unfortunately his teachings were lost until the mid 17th century when a
physician rediscovered the different types of malarial fevers and found that an
extract from a Peruvian bark (quinine) could cure the disease. Ancient human
remains in Chile show clear evidence of Chagas Disease, which is caused by a
protozoal parasite, Trypanosoma cruzi (see below).
Protozoal parasites could not be discovered until the invention of the
microscope.
But discoveries in science require not only
technology, but also a
conceptual framework or paradigm,
which allows predictions.
For example, Robert Hooke in 1665
developed a microscope with 30X magnification and saw "cells" in slices of cork
(actually cell walls). This observation made no impact on science at all. He
however did influence Antony van Leeuwenhoek to begin grinding lenses!
In the later part of the 17th century, a Dutch amateur scientist named Anton van
Leeuwenhoek made some really fantastic discoveries - but they made
essentially
no impact on science. Using tiny single-lens microscopes of
his own design,
Leeuwenhoeck made the first descriptions of protozoa, bacteria, and
spermatozoa which he called "animalcules" and made the first detailed
descriptions of the red blood cell. He wrote letters to the Royal Society in
London from 1673 to 1700, which were published. He looked at everything - rain
water, canal water, his saliva, his feces. He saw an entirely new world which
had never been seen before. He also discovered the first protozoal parasite -
Giardia.
Let me give you one anecdote - Leeuwenhoek was proud of his white teeth -
he rubbed them with salt, picked with a toothpick and rubbed again. But he still
had white matter which when suspended in water had many small animals in
motion=bacteria. He looked at the white matter from the teeth of a man who did
not drink or smoke and an alcoholic who only rinsed his mouth with wine. The
sober man had spiral-shaped bacteria whereas the alcoholic had no bacteria! The
moral is obvious!
It would seem unbelievable that an amateur could see things that
professional scientists of the day could not see. The reason is that he could
avoid the problems of chromatic and spherical aberration by using only a single,
high-power, quality lens. In the compound microscopes of the time, these errors
are synergistically increased. Click here to see
the Leeuwenhoek web site.
A recent study of the remaining Leeuwenhoek microscopes and
several reconstructions shows
their magnifications to be from 50X over 200X, with resolutions as good as 2
microns. The microscope must be held close to the eyes and is very hard to
focus. It requires excellent eyesight. Plans are
available for making your own reconstruction together with instructions
for using the microscope!

A
picture of one of Al Shinn's reconstructions. |
|
Leeuwenhoeck was also the first to cut thin sections for microscopy. He also
was quantitative - he measured sizes in comparison to sand grains and counted
the objects by using a capillary tube. His sizes were very accurate. In 1980,
several packets he sent to London were discovered and the material reexamined by
modern microscopy by Brian Ford.
Following are figures showing these samples.

Specimens from Leeuwenhoeck attached to letters he sent to the Royal
Society - rediscovered by Brian Ford in 1980 |

From Darnell, Lodish and Baltimore, Molecular Cell
Biology, Freeman and Co., 1990 |
BUT - his discoveries made no impact on science. One reason for this is that the technology was not
available to other scientists and the more important reason is that there was no
existing theory or "paradigm" into which these observations would fit.
Leeuwenhoek himself called the organisms he saw "little animals" since there was
no concept yet that the cell was the basic unit of life and that some organisms
were actually single cells.
An important digression
The scientific method is to make observations and then to fit
these into an existing theory. This then leads to predictions of new
observations which can verify or negate the original theory. A scientific "fact"
is an observation that fits into a paradigm and leads to new predictions, that
is, is verifiable. Changing a paradigm is a rare event in science and creating a
new paradigm is even more rare. Let me give you some examples of what are
considered paradigms. The cell theory discussed below is one and I would include in
the cell theory paradigm all recent studies on molecular and cell biology. The
discovery of the double helix of DNA and the realization that this
explained replication of genes created the conditions for people to reevaluate the
papers of Avery and led to a new paradigm - that DNA was the genetic material. The theory of evolution was a paradigm
created by Darwin and Wallace and has so thoroughly permeated modern biology
that every new fact is interpreted through evolution.
Advances in microscope technology:
1823 - The achromatic lens was developed - led to a 1 µm resolution.
This led to the discovery of the cell nucleus by R. Brown in 1833, the
nucleolus in plant cells by Schleiden, cells in animals by Schwann, mitochondria
in 1858 by Koelliker - and finally to a conceptual change in
1839:
The Cell Theory by Schleiden and Schwann.
Another conceptual change occurred in 1858 - Virchow stated that
cells come from cells and each cell is an organism.
New technology was rapidly developed:
1878 - Oil Immersion lens
Led to
discovery of chromosomes by Flemming in 1879.
1886 - Apochromatic lens -
resolution of 0.2 um = theoretical limit of light microscope.
Figure from Darnell, Lodish and Baltimore, Molecular Cell Biology, Freeman
and Co., 1990
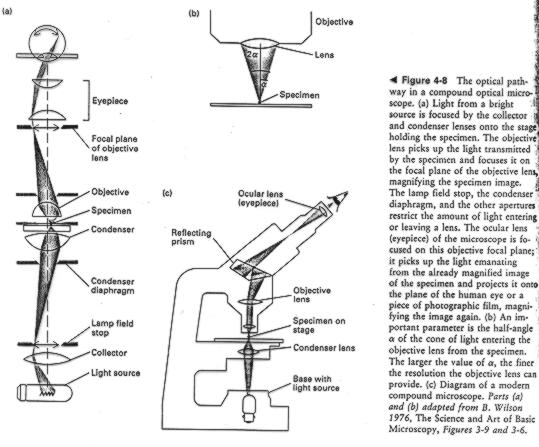
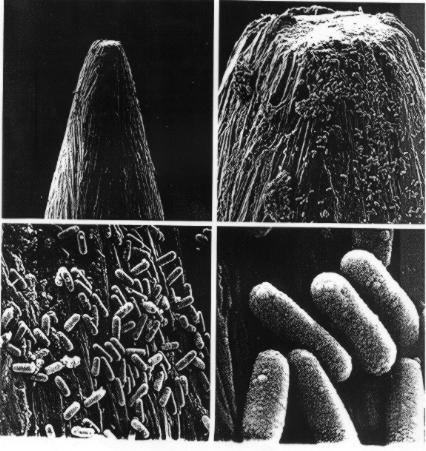
The head of a pin at different levels of resolution (from Darnell, Lodish
and Baltimore, Molecular Cell Biology, Freeman and Co., 1990)
Other major conceptual advances included the proof by Pasteur that
spontaneous generation did not occur and the discoveries by Koch that
microorganisms caused diseases. Finally, Patrick Manson introduced the concept
of vector-borne parasitic diseases and founded the field of Tropical Medicine,
from which modern parasitology was derived.
Patrick Manson

After receiving his MD degree in 1860, Manson went to work in Formosa and
then China. In 1877 he learned of the discoveries by Bancroft of the Filarial
worm in blood and tissues of a patient with elephantiasis disease. Manson found
these worms in normal patients also and saw that they had a daily periodicity
that was similar to that of the feeding behavior of mosquitoes. He tested his
theory that mosquitoes carried the worms by allowing them to feed on an infected
person; the worms then developed in the insect. This was a major advance in
medicine, that a specific blood sucking insect was an intermediary in the
propagation of a particular disease. In 1898 Manson was convinced by Ronald Ross
that mosquitoes could possibly transmit malaria. To test this theory he actually
allowed Anopheles mosquitoes which had fed on a tertian malaria patient to bite
his own son and his son came down with malaria! He then went with some friends
to the Roman Compagna near Ostia, which was notorious for malaria, and lived in
a mosquito-proof hut from July to October; he and his friends remained in
perfect health, although, I imagine, somewhat bored. These experiments
established clearly that mosquitoes carried malaria.
In 1898 Manson published the Manual of Tropical Disease, which established
this as a field of science, and he later founded the London School of Tropical
Medicine and Hygiene. The science of parasitology emerged from the medical
discipline of Tropical Medicine. It came to be limited to the study of parasitic
protozoa (eukaryotic cells), helminths and arthropods, and did not include the
study of bacteria, fungi or viruses, which could also be classified as parasites
by our above definition. This illustrates the facts that scientific disciplines
are in a basic sense arbitrary attempts to organize natural phenomena and that
these attempts are molded by history and influential people.
William Leishman and Charles Donovan
and the discovery of the cause of Kala Azar
Kala Azar or Black Fever probably existed for centuries in Bengal and China,
but the first recognized outbreak was in 1824 in Jessore, India. It caused the
deaths of 750,000 people in 3 years. It was thought to be a type of malaria with
relapses, emaciation, enlargement of liver and spleen. It appeared to be
communicable and spread slowly along traffic routes.
Leishman grew up in Glasgow and became a doctor in the British Army Medical
Service in1887. He was sent to India where he studied kala azar and then
returned to London in 1900. His first original contribution to science was the
development of an easy method to stain blood for malaria parasites, which is
still used today. In 1901, he stained spleen smears from a soldier dying of kala
azar, and observed small bodies, 2-3 um in size, each with two stained objects -
spherical and rod-shaped, and he published this in 1903. These were the
amastigote forms of Leishmania donovani. Also in 1903, Donovan, who was a
Professor at Madras University in India, sent a sketch of parasites he saw in
the spleen of a patient suffering from spleen enlargement and fever to Ronald
Ross. Ross realized that these were the same parasites which Leishman had seen
and he created the genus, Leishmania, in their honor. The parasites for
some time were called "Leishman-Donovan" bodies, but are now called
"amastigotes".
Click here
to read more about William Leishman.
Soon after this, the protozoa were cultured in a blood medium, but the
parasites had flagella and resembled trypanosomes.
The search for the vector of Leishmania donovani illustrates the way
science sometimes works. Donovan and others suggested that bed bugs were the
vector and this led to many years down a blind alley. The evidence that sand
flies were the vector came mainly from a coincidence of the range of the insect
and the range of the disease. H.E. Shortt was convinced that sand flies were the
vector, but definitive proof was lacking. Finally in 1939, R.O. Smith found that
flies which were allowed to feed on raisins after their blood meal became
clogged with parasites. The Leishmania promastigotes grew so well that they
plugged the pharynx and this proved to be how they were injected into the host
at the next blood meal. As quoted in Desowitz's book from a lecture by a Dr.
Knowles:
"The story of the discovery of how kala azar is transmitted from man to man
is one of the most amusing, also perhaps one of the sorriest in tropical
medicine. It is a history of almost 20 years of wasted effort..of false starts
and erroneous conclusions; of acute controversies and the flow of much ink; of
wasted effort and the absence of coordinated inquiry."
David Bruce

David Bruce was born in Melbourne, Australia, in 1855. He grew up in
England and graduated in medicine in 1881. He joined the Army Medical Service in
1883. In 1988 he spent a leave working in Koch's laboratory. He was then sent to
South Africa where he discovered that a new trypanosome (T. brucei)
was the cause of a disease of domestic animals
called Nagana. Bruce described the disease as
follows:
"The
horse stares, he has a watery discharge from his
eyes and nose. Shortly afterwards a slight
swelling of the belly and puffiness of the sheath
may be noticed, and the animal falls off in
condition. The hind extremities also tend to
become swollen; and these various swellings
fluctuate, one day being less marked, or having
disappeared. During this time the animal is
becoming more and more emaciated, he looks dull
and hangs his head, his coat becoming harsh and
thin in places; the mucous membranes of the eyes
and gums are pale, and probably slight cloudiness
of the cornea is observable. In severe stages, a
horse presents a miserable appearance. He is a
mere scarecrow, covered by rough hair, which falls
off in places. His hind extremities and sheath may
be more or less swollen, sometimes to a great
extent, and he may become blind. At last he falls
to the ground and dies of exhaustion. During his
illness he has shown no symptoms of pain, and up
to the last days has had a good appetite."
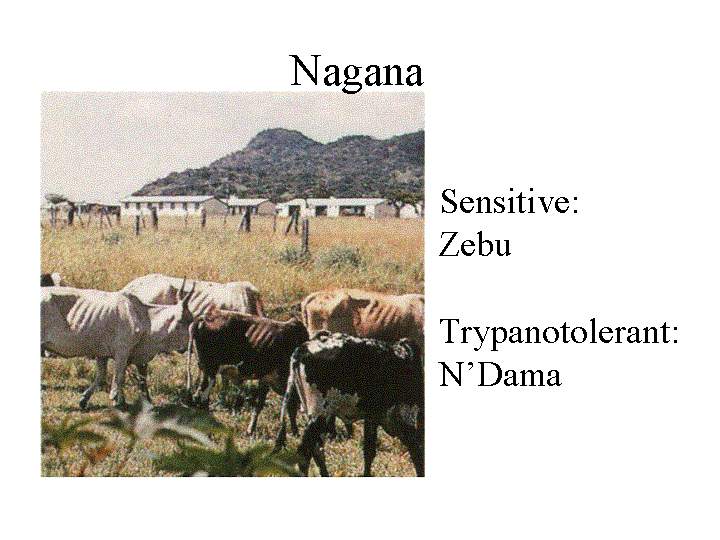
Bruce found the
organism in the blood and showed that they were
transmitted from antelopes to cattle by the tsetse
fly, Glossina morsitans. Bruce was the
first to prove that an insect carried a protozoan
causing a parasitic disease.

In 1903 he went to Uganda to investigate human
Sleeping Sickness. Sleeping sickness is a
disease that was widespread in Africa. Victims
have a prolonged debilitating fever and after a
few months become confused and stuporous. They
often may develop enlarged lymph nodes and spleen,
and ultimately they relapse into a coma and die.
Post-mortem examinations show pericardial and
peritoneal effusion and evidence of meningitis.
Dr. Aldo Castellani, another member of the
commission, had found trypanosomes in the
cerebrospinal fluid of sick patients. Bruce found
that trypanosomes were
present both in the blood and late in the
infection in cerebrospinal fluid
in all Sleeping Sickness patients. He also was
able to infect monkeys with spinal fluid and the
monkeys developed sleeping sickness. Bruce
also noticed a co-incidence
of the distribution of the tsetse fly and the
disease. He organized a national tsetste fly
hunt and thousands of tsetse flies were dissected
to study the development of trypanosomes and their
evolution. Tsetse flies were allowed to feed on
animals or persons suffering from trypanosomal
disease and then on other animals to see if the
disease had been transmitted. Bruce published his
findings in 1903. From 1908 to 1910, Bruce
rejoined the Royal Societies continuing commission
in Uganda, where he directed research into
conditions for transmissibility of T. gambiense by Glossina palpalis, and studied cattle
and game as potential reservoirs of the parasites.
The commission was closed when one of its members,
an unfortunate Lieutenant Forbes Tulloch, died of
the disease.
In 1911, Bruce investigated an epidemic amongst
the natives of Kaviondo on the shores of Lake
Nyasa, whose banks swarmed with flies. Flies
caught by the native boys (2 of whom died of the
disease) were put in cages in the laboratory and
then fed on three healthy animals: a dog, a
monkey and a goat, each animal receiving 180
flies. In 56 experiments using 10,000 flies, 9
monkeys, 14 dogs and 11 goats were infected – an
infectivity rate of 1/500. Thus, an inhabitant had
a 1/500 chance of being bitten by an infected fly.
Bruce received many
honors but his abrupt manner,
blunt speech, and egotistical personality made him
many enemies. A famous surgeon, Harvey Cushing,
remembered Bruce as a member of a research
committee they both served on: "General Sir
David Bruce sank into a divan, stretched out his
highly polished boots into the middle of the room,
inserted his spurs into the rug, drew his John
Bull visage deep into his clothes, turtle fashion,
and slept profoundly which was good for the
general and also helped the meeting."
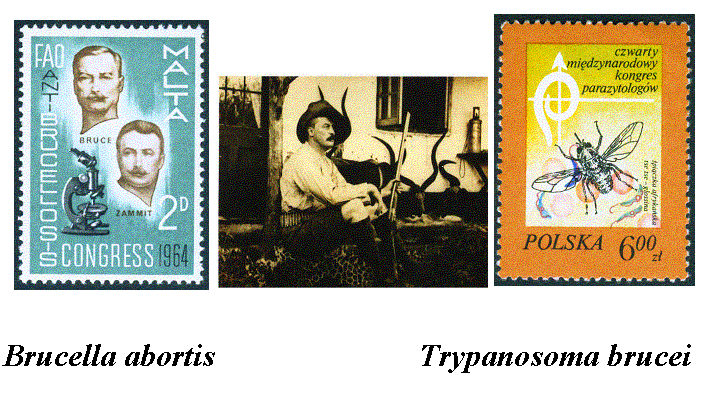
In the center is a photo of Bruce in his
hunting gear. His fame led to the issuance of stamps in his honor in several
countries.
Click
here for a good review of Bruce's life.
Charles Laveran
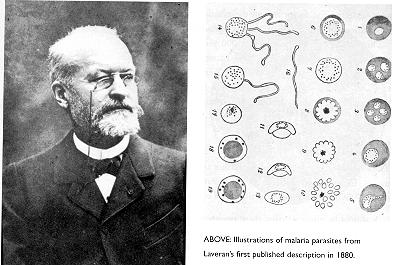
Charles Laveran was a French Army surgeon, who in 1880 examined the
blood of a soldier suffering from malaria in Algeria detected movement of the
red blood cells and found that this was due to fine filaments attached to round
pigmented bodies. He published that these were parasites but the conceptual
framework of the time was that malaria was caused by a bacterium from swamp
water, and noone believed his theory. In 1882 Laveran visited Rome and showed
his parasite to two scientists, Marchiafava and Celli. In 1884 they looked at
unstained blood from malarious patients and saw a active amoeboid ring
(trophopzoite) in the red blood cells. They published this finding and named it
Plasmodium malariae, but did not refer to Laveran since they thought it was
something different from what he showed them. Camillo Golgi in Pavia also was
studying malaria and showed that the clinical features were correlated with the
life cycle of these parasites. He recognized several different species with
different periodicities. Finally several other Italian workers discovered P.
falciparum, which had a 48 hour cycle and produced a deadly disease.
Ronald Ross
 Ross was born in India and went to Medical School in London. He
joined the Madras Medical Service and became interested in malaria. Ross showed
in 1895 that the parasites Laveran had seen underwent exflagellation in
Anopheles mosquitoes. In 1897 he tried breeding mosquitoes in the lab and fed
them on a patient with malaria. On August 20 he saw pigmented oocysts in the
wall of the stomach of one of these insects which had been fed 4 days earlier.
Ross then turned to the study of bird malaria. Several Italian scientists
carried on the study of human malaria. Ross unfortunately had a long feud with an Italian scientist, Grassi, on who
actually discovered the life cycle of Plasmodium. This, unfortunately, is also
the human side of science.
Ross was born in India and went to Medical School in London. He
joined the Madras Medical Service and became interested in malaria. Ross showed
in 1895 that the parasites Laveran had seen underwent exflagellation in
Anopheles mosquitoes. In 1897 he tried breeding mosquitoes in the lab and fed
them on a patient with malaria. On August 20 he saw pigmented oocysts in the
wall of the stomach of one of these insects which had been fed 4 days earlier.
Ross then turned to the study of bird malaria. Several Italian scientists
carried on the study of human malaria. Ross unfortunately had a long feud with an Italian scientist, Grassi, on who
actually discovered the life cycle of Plasmodium. This, unfortunately, is also
the human side of science.
Following is an interesting description of the discovery of Ross from a book
by Foster:
A selection from W. D. Foster, A History of Parasitology, E.S. Livingston Ltd.,
London (1965)
History of Parasitology, E.S. Livingston Ltd.,
London (1965)
….to the Director-General hoping to get seconded for special duty. After his
severe labour at Otacammund he felt a " violent reaction against the microscope
" and could hardly bring himself to look through it for a month. The monsoon
appeared to have failed and the heat and dust seemed unbearable. He wrote:
What ails the solitude? Is this the judgment day? The sky is red as blood;
The very rocks decay And crack and crumble, and There is a flame of wind
Wherewith the burning sand Is ever mass'd and thin'd.
Then the thought struck him: " Why not see whether mosquitoes fed on
malarial blood as before contain any of the mosquito-parasites which I had found
in the Sigur Ghat? I was at full work again on 21st July, 1897 on the last lap
".
His principle of work was, as he wrote to Manson, as follows: " I am of
course sticking to the flagellulae again but on a slightly modified principle.
Owing to their extreme delicacy I doubt whether they can be directly followed
into the insect tissues; but this difficulty can be avoided in a simple manner.
The question I have set myself to answer, and which I now feel able to tackle,
is " Do healthy mosquitoes fed on malarial blood, contain any parasite which
similar mosquitoes fed on normal blood do not contain? ... I keep mosquitoes,
after feeding on crescents, for one, two, three days and then examine, so as to
allow the flagellulae time, by supposition, to mature into full-grown
mosquito-parasites suspicious bodies being noted, I have only to compare with
control insects . . . ".
Ross must be allowed to tell his own story: " But the weather became very
hot again in August. At first I toiled comfortably, but as failure followed
failure, I became exasperated and worked till I could hardly see my way home
late in the afternoons. Well do I remember that dark hot little office in the
hospital at Begumpett, with the necessary gleam of light coming in from under
the eaves of the veranda. I did not allow the punka to be used because it blew
about my dissected mosquitoes, which were partly examined without a cover glass;
and the result was that swarms of ' eye-flies '-minute little insects which try
to get into one's ears and eyelids-tormented me at their pleasure, while an
occasional Stegomyia revenged herself on me for the death of her friends. The
screws of my microscope were rusted with sweat from my forehead and hands, and
its last remaining eye-piece was cracked ". Writing to his wife he said: " I
have failed in finding parasites in mosquitoes fed on malaria patients, but
perhaps am not using the proper kind of mosquitoes ".
But now " some Angel Fate " had put into the hands of one of his "
mosquito-men " some larvae, which hatched out a different, dappled mosquito, a
single specimen of which Ross had seen in Sigur Ghat. On August 16th these were
fed on his malaria patient, Husein Khan. That evening he wrote to his wife: " I
have found another kind of mosquito with which I am now experimenting, and hope
for more satisfactory results with it ".

On the 17th he dissected two of these
mosquitoes but found nothing unusual. On the 19th he killed another and found "some peculiar vacuolated cells in the stomach about 10 microns in diameter ". He
thought little about them and was rather disheartened at his results with the
new species. On August 2oth, a dull, hot day, Ross went to the hospital at 7
a.m., examined his patients, dealt with his correspondence and had a hurried
breakfast in the mess. One of his mosquitoes had died and this he dissected
without noting anything significant. He had two mosquitoes left of the batch fed
on Husein Khan on the 16th and at about 1 p.m. he determined to sacrifice one.
Dissecting it he scrutinized the tissues micron by micron, when suddenly, in the
stomach wall he "saw a clear and almost perfectly circular outline before me of
about 12 microns in diameter. The outline was much too sharp, the cell too small
to be an ordinary stomach-cell of a mosquito. I looked a little further. Here
was another and another exactly similar cell ". He changed the focus of his
microscope and there within each of these new cells was a cluster of black
pigment. He made rough drawings in his notebook, sealed his specimen, went home
to tea and slept solidly for an hour. The pigment puzzled him, for the flagella
contained no pigment, but the thought struck him that if the cells were really
parasites they should grow in size in the last remaining mosquito during the
night. He spent the night in agony lest his last remaining mosquito should die
and decompose before morning. Next day he slew and dissected, with shaking hand,
this remaining specimen. There were the cells again, twenty-one of them, just as
before, only now much larger . . . The cells were therefore parasites, and, as
they contained the characteristic malarial pigment, were almost certainly the
malaria parasites growing in the mosquito's tissues. The thing was really done.


Oocysts of malaria parasites in the midgut wall of a mosquito.
Ross's notebook Aug. 20, 1897.
Next morning, after writing to his wife, Ross scribbled in a notebook:
This day designing God
Hath put into my hand
A wonderous thing. And
God
He praised. At his command
I have found thy secret deeds
Oh
million-murdering Death.
I know that this little thing
A million men will
save
Oh death where is thy sting?
Thy victory oh grave?
All this he reported in a letter to Manson dated August 22nd,
Ross had, still, a long way to go before the life-cycle of the malaria
parasite was worked out but the great significance of his discovery on August
20th-Mosquito Day, as he ever after called it was that he had shown that the
malaria parasite went through further development in the mosquito tissues,
beyond the crescent-spherule flagella transformation, which occurred, at least
to some degree, on a microscope slide. Immediately following these discoveries
his work was held up by shortage of dappled-winged mosquitoes and on 4th
September he joined his family at Bangalore for 10 days leave. He made it his
first task to write a paper " On some peculiar pigmented cells found in two
mosquitoes fed on malarial blood ". The article appeared in print in the
British Medical journal on December 8th, 1897. Meanwhile he returned to
Secunderabad and after some delay obtained a supply of mosquitoes bred from the
larvae.

There is a recent review of
Ronald Ross in Science magazine. Worth reading!
Carlos Chagas

Carlos Chagas was a Brazilian scientist who was hired to eradicate an
outbreak of malaria that occurred during the construction of a railroad in
Brasil in 1907.
While he was working on this, people told him about a Reduviid bug, the
barbeiro, that lived in rural huts and would come out at night and bite
people on the face. Chagas found large numbers of flagellates in the hind gut of
these insects. He allowed them to bite lab animals and they became infected in
3-4 weeks. He showed that armadillos were the natural reservoir. The parasites
could be easily cultured on blood agar. He called these Schizotryanum cruzi
and today are called Trypanosoma cruzi. Then Chagas discovered the
trypanosomes in the blood of patients. He identified an acute phase and a
chronic phase and showed that there was a cardiac involvement.
In 1911-1913 Chagas made several expeditions to the Amazon region to
investigate tropical diseases which were hindering the building of railroads.
These expeditions led to increased knowledge about Chagas Disease and malaria in
the Brazilian Amazon.

This was a unique case of someone first discovering a parasite and its life
cycle, and then discovering the disease caused by the parasite and working out
all the details of the pathology and epidemiology of the disease. It was a real
tour-de-force in science.
William Trager

William Trager was a Professor at Rockefeller University in New York.
He worked on protozoal parasites for his whole career - beginning with
termite gut protozoa and then with malarial parasites. He was fascinated by the
intracellular growth of Plasmodium and decided more than 20 years ago that the
ability to continuously culture malarial parasites would not only lead to
understanding the reasons for this unusual habitat, but also was a prerequisite
for the ultimate goal of obtaining a vaccine against malaria. For many years
Trager painstakingly attempted to culture the avian malarial parasite, P.
lophorae, free of red blood cells. He was able to obtain several cycles of
development and learned much about the nutritional requirements of these
parasites. Then in 1976, Trager and Jensen discovered a simple method to grow
the pathogenic human malaria, P. falciparum, in continuous culture inside
red blood cells. This breakthrough stimulated a huge amount of research and may
yet lead towards the development of a vaccine.
Dr. Trager at work:

I quote from an
article
about his work:
In 1973, Trager wrote a journal article that in essence
announced his resignation from a 30-year attempt to grow malaria in culture. It
couldn't be done, he said, and he would probably turn his efforts elsewhere.
Without the ability to grow malaria in the laboratory, prospects were dismal
that a vaccine could be created or new drugs developed to treat the disease.
Researchers instead would require ready access to malaria patients, which meant
living in Africa and braving the concomitant political and physical challenges.
With the grandfather of malaria research surrendering the battle and approaching
retirement age, it appeared a tiny microbe had routed the scientific world.
But in 1975, with malaria cases again rising after a
two-decade campaign to eradicate it initially appeared to be succeeding and then
collapsed, the World Health Organization (WHO) and USAID implored Trager to try
again. He relented and agreed, but this time asked for help from a young
graduate student who was effectively culturing a related organism that causes
disease in animals.
The student, Jim Jensen, was just finishing his
doctorate at Auburn University in Alabama (after completing a BS and MS in
zoology at BYU, then beginning his doctorate at Utah State University but
transferring to Auburn after the death of a key Utah State professor). Jensen
quickly wrapped up his dissertation and moved with his young family to New York
City, where he joined Trager in his Laboratory of Parasitology at the
prestigious Rockefeller University.
Within one year, Trager and Jensen had triumphed over
their nemesis, finally growing malaria successfully in culture. The duo's 1976
article in the top-flight journal Science provided the formula. It also made
news worldwide, including the front page of the New York Times. The two
scientists had given malaria research a new life.
"Researchers don't need to go to Africa anymore," said
Jensen, speaking in his large laboratory on the top floor of the Widtsoe
Building. "All you need is an incubator. You can grow malaria in any
laboratory."
And grown it is, in hundreds of labs around the world,
including Jensen's. Today, the Science article is so classic that it is rarely
cited formally. Instead, researchers simply report that they cultured the
malaria parasite "according to standard procedures."
Dr. Trager died Jan. 22, 2005. Following is a Memoriam in
press in J. Eukary. Microbiol.:
IN MEMORIAM: WILLIAM TRAGER (1910-2005)
William Trager, Professor Emeritus at the Rockefeller
University died at his home in Manhattan on January 22, 2005. Professor Trager
during his long and productive career made many seminal contributions to the
“how and why” of organisms living together. Now, with his passing it is fitting
that we pay respect to a brilliant researcher, a thoughtful adviser and an
inspirational teacher.
William Trager was born on March 20th 1910 in
Newark, N.J. the son of Leon and Anna (Emilfork). Early on his parents
encouraged scholarly and intellectual pursuits. He received his B.S. degree from
Rutgers University in 1930 and his M.A. and PhD from Harvard University in 1931
and 1933 respectively. After completing his PhD under the direction of L.R.
Cleveland where he studied the cellulase activity of termite flagellates he took
a position as a Fellow of the National Research Council in the Department of
Animal Pathology in the Princeton Division of the Rockefeller Institute. There,
in the laboratory of R. W. Glaser—whose forte was growing axenic (bacteria-free)
cultures of protozoans—Trager worked on insect physiology and pathology. This
early training sowed the seeds for his talent and success in culturing parasitic
protozoans. In 1934 he was appointed to the staff of the Rockefeller Institute
where he remained for his entire professional life.
All of Professor Trager’s research centered on three
questions:
- What special environmental factors does a host supply
to a parasite?
- Can these factors be furnished in a non-living culture
medium so that the parasite will go through its complete developmental and
reproductive cycle?
- How do the products of the parasite effect appropriate
modifications in the host?
Answers to these questions, he
believed, would lead to new chemotherapeutic agents, protective vaccines and
provide a molecular basis for understanding parasitism.
During Professor Trager’s early period at Rockefeller he
succeeded in growing mosquito larvae in axenic culture—no small feat since this
was before the advent of antibiotics—and this allowed for a determination of
their nutritional requirements. Another accomplishment was his ability to
propagate a polyhedral virus in silkworm tissue cultures and to achieve the in
vitro multiplication of Western equine encephalitis virus in mosquito
tissues—the first demonstration of an arbvovirus in insect tissue culture.
Trager would return to insect tissue cultures in 1958-59 and 1975 when he used
tsetse fly tissue cultures for growing the insect stages of Trypanosoma vivax.
Trager’s interests in exploring the host-parasite relationship led to his 1939
discovery of acquired immunity to ixodid ticks.
Professor Trager’s earliest contributions to malaria came
as a result of his World War II experiences in New Guinea as an Officer in the
U.S. Army Sanitary Corps. There, along with Frederick Bang and Malcolm Ferguson,
he was involved in the supervision of trials of a new antimalarial, atebrine.
Upon is return from the Pacific, he began his laboratory studies at Rockefeller
on the nutritional requirements of the malaria parasite using the bird malaria
Plasmodium lophure, which attained very high parasitemias in white Pekin
ducklings. After 4 years of painstaking effort Dr. Trager published a series of
papers on the extracellular cultivation of the asexual stages of this
parasite—from ring to schizont. This was a Herculean effort that defined ATP and
coenzyme A, malate and pyruvate as well as high K+ as key requirements. He went
on, in subsequent studies, to define the role of pantothenate, folic and folinic
acid and biotin in the growth and maturation of the parasite. During the early
“lophurae” period he worked with R. Barclay McGhee in describing erythrocyte
receptors for the merozoite---studies later pursued by Louis Miller, Margaret
Perkins, Terrence Hadlley and Geoffrey Pasvol that led to the identification of
glycophorin and Duffy factor as receptors for P. falciparum and P.
vivax respectively. In 1957, now at the Rockefeller Institute in New York
City, Trager initiated, in collaboration with Dr. Maria Rudzinska, a cell
biologist, electron microscopic studies of the intracellular development of
Plasmodium and Babesia. Rudzinska and Trager discovered the
parasitophorous vacuolar membrane, the large hemoglobin-containing food
vacuoles, and described the mitochondrion and nucleus of P. lophurae.
Although such work was later exceeded in refinement of detail by others (such as
Susan Langreth, Lawrie Bannister, Masamichi Aikawa and Norbert Lanners) it is
well to recognize that this pioneering work was carried out before there were
better fixatives such as glutaraldehyde, and involved transmission electron
microscopes with far less resolution than those in use today. In 1966, Trager in
collaboration with Rudzinska and Phyllis Bradbury described the ultrastructure
of P. falciparum and identified the knobs on the surface of the infected
erythrocyte that contribute to sequestration. Later studies (1968) with the
monkey malaria, P. coatneyi, confirmed much of this, and subsequently
with Susan Langreth (1978) the fine structure of extracellularly grown parasites
was described.
In 1976 Professor Trager literally invented a method for
the continuous cultivation of the erythrocytic stages of P. falciparum.
The work was elegant in its simplicity: a stationary layer of red cells, a
medium containing human serum, a buffer to prevent pH shifts from lactic acid
produced by the parasite, and a gas mixture low in oxygen and high in carbon
dioxide. Initially developed by Trager as a continuous flow system it was
modified through collaboration with a postdoctoral fellow, James Jensen, to one
that is completely portable---the candle jar technique. The successful culture
of P. falciparum spawned a renaissance of research on the immunology,
cell biology and molecular biology of this parasite. Since the publication of
this monumental discovery there has been an explosion of information about
falciparum malaria. The availability of “tamed” falciparum allowed this
parasite to be grown anywhere in the world without sophisticated equipment, and
allowed for new knowledge about the genetics of red cell susceptibility, the
antigens of the parasite, how to achieve differentiation into gametocytes, the
number of chromosomes, the sequencing of the genome, the development of knobless
lines, the testing of parasites for drug sensitivity and much more. Trager
developed well-characterized clones of P. falciparum and studied
gametocyte formation in some of these clones (1984). And, his success in the
extracellular development of erythrocytic merozoites of P. falciparum to
the ring stage, suggested that invasion of the red cell per se need not be a
trigger for differentiation.
In his 1962 “Address of the Past President of the Society
of Protozoologists”, published in the Journal of Protozoology---which he was
instrumental in founding---Trager spoke not a word about malaria but confined
his remarks to the “differentiation exhibited by protozoa with reference both to
their own intrinsic interest and especially to their value in the study of
cellular differentiation.” This address was illustrative of his general
interest in protozoa and their utility as model systems to address basic
biologic problems.
Another major research interest was the kinetoplastid
protozoa. In 1952 Trager showed that the normally intracellular amastigote forms
of the human parasite, Leishmania donovani could be cultivated
extracellularly for several days at 370C. This work was stimulated by
Trager’s close friendship with Leslie Stauber at Rutgers University who was the
doyen of pathogenic Leishmania at that time. This paper provided the
stimulus for the eventual development of continuous culture systems for
Leishmania amastigotes in several other laboratories. In 1958, Trager spent
a sabbatical year in Nigeria at the West African Institute for Trypanosomiasis
Research studying T. vivax, the most important pathogenic trypanosome of
cattle in West Africa. He mentioned in his Presidential Address to the American
Society of Parasitologists that earlier he had been told by Dr. Robert Hegner
that “if you want to see the world…become a parasitologist” and he considered
this to have been sound advice. In Nigeria, Trager combined his previous
expertise in insect tissue culture with his knowledge of culture conditions and
established the complete life cycle of T. vivax using tsetse fly tissue
cultures. He returned to Nigeria in 1973-74 and after initially obtaining
negative results, probably due to strain differences of the parasites, quickly
retooled and established a method to culture bloodstream forms of T. vivax
using tstetse organ cultures.
In 1957 Trager developed a defined chemical medium for the
continuous culture of the lizard parasite, L. tarentolae. This was the
first culture of a hemoflagellate in a defined medium and allowed Trager to
define several metabolic and nutritional requirements of these parasites such as
the vitamin biopterin. This finding led to much recent work on selective
chemotherapy of these parasites in other laboratories. In collaboration with the
electron microscopist, Maria Rudzinska, Trager then showed that acriflavin-induced
akinetoplastic or dyskinetoplastic L. tarentolae cells exhibited major
changes in the DNA-containing kinetoplast at the base of the flagellum. This
work was the impetus for much research in many laboratories on the mitochondrial
DNA of trypanosomes (including the PhD thesis research of one of the authors,
LS). And finally, in 1979, Trager found a graduate student, Michael Yamin,
interested in his old love, termite protozoa, and they showed that
Trichomitopsis termopsidis contained cellulase activity that was not
dependent on extracellular or intracellular bacteria. Defaunating and
refaunating termites with these protozoa and following the effect on their
longevity demonstrated the role of these protozoa in the nutrition of the
termite.
Professor Trager, the exemplar of a bench scientist,
worked in his Rockefeller laboratory on a daily basis, carrying out with his own
hands the preparation of reagents, setting up of cultures, carrying out unbiased
analysis, and meticulously recording his observations and conclusions. The
impeccable thoroughness with which he pursued his science resulted in his work
having a high degree of reproducibility and reliability. Yet through it all he
was always modest. At Rockefeller there was a generous exchange of ideas, of
methods, and even of ducklings; there was respect for one another, and each of
us was made to feel as if we were colleagues. Some of us felt uncomfortable
calling our mentor “Bill” but it was encouraged by him and there was enduring
warmth for each of us. That support extended from the very first day we entered
his laboratory to the time of his death. Although Professor Trager encouraged
students and post-doctoral fellows to seek out independent positions he expected
that we would carry with us that which we learned at Rockefeller. However, most
of us felt that we never really left the Trager lab for his support and high
standards were always with us. He enriched our lives beyond measure.
During his lifetime Professor Trager received many awards
and honors. He received honorary degrees from Rockefeller University (1987) and
Rutgers University (1965), the Manson Medal of the Royal Society of Tropical
Medicine and Hygiene (1986), the Leuckart Medal (1982), S.T. Darling Medal of
the World Health Organization (1980), the Augustine Le Prince medal of the
American Society for Tropical Medicine and Hygiene (1991) and Thailand’s Prince
Mahidol Award (1994-1995). In 1973 he was elected to the National Academy of
Sciences (USA) and in 1973-74 was a Guggenheim Fellow. He served the Society of
Protozoologists as Editor (1953-65) and President (1960-61), was President of
the Society of Parasitologists (1973-74) and President of the American Society
of Tropical Medicine and Hygiene (1978-79).
Professor Trager’s contributions to malaria and
kinetoplastids are represented by more than 200 publications, two books (“Living
Together: the Biology of Animal Parasitism”, Plenum 1986 and “Symbiosis”
Rheinhold, 1970) and many honors. However, Bill Trager did much more than that;
he served as the guiding light for a large number of malariologists including:
Barclay McGhee, Ira Singer, Wassim Siddiqui, George Jackson, Peter Bennett,
Edward Platzer, James Jensen, Susan Langreth, William Scheibel, Robert Reese,
Araxie Kilejian, Ann Vezza, Phyllis Bradbury, Harold Stanley, Margaret Perkins,
Milton Friedman, Norbert Lanners, Fred Brohn, Chariya Brockelman, Mary Motyl,
Julie Olsen, V. Bhasin, Fred Gyang, Robert Herman, Michael Hollingdale, Phuc
Nguyen Dinh, Jonathan Williams, Gokal Gill, and Irwin Sherman. And he sparked
interest in kinetoplastids by having as associates or trainees Philip
D’Alesandro, Curtis Patton, Stuart Krassner, Larry Simpson, Dennis Dwyer, Andrew
Balber, K.P. Chang, Alan Clarkson, Jan Keithly, Miki Rifkin, Rolf Steiger and
Phyllis Straus. Others in his laboratory who worked on symbionts, parasites and
insects were Earl Weidner, Joseph Peleg, Abraham Held, Dunne Fong, Wallace Fish,
Dickson Despommier, Steve and Marlene Karakashian, and Michael Yamin.
Professor Trager was a member of many scientific societies
including the American Association for the Advancement of Science, New York
Academy of Sciences, Entomological Society of America, American Society of
Zoologists, American Society of Parasitologists, American Society of
Experimental Pathology, Society of Experimental Biology and Medicine, American
Society of Tropical Medicine and Hygiene, Royal Society of Tropical Medicine and
Hygiene. He enjoyed summer stays at the Marine Biological Laboratory in Woods
hole, MA and was a member of the Corporation.
William “Bill” Trager was generous in providing the
resources of his laboratory for training, sent his cultures to all who needed
them and was unstinting in his devotion to his students and colleagues. Those of
us who were fortunate enough to be friends of this very modest man will remember
him with pleasure and affection.
Irwin Sherman and Larry Simpson (2005).
Modern Parasitology
In recent years the study of parasites has had a resurgence of interest due
to the development of recombinant genetics and cell and molecular biology and
the ability to study parasites at these levels. There still is a close
attachment of parasitology with Tropical Medicine since most of the parasitic
diseases are found in the tropics and subtropics. The World Health Organization
has recently stated that there are six major human tropical diseases -
schistosomiasis, malaria, filariasis, African trypanosomiasis, leishmaniasis,
and leprosy. The first five of these are caused by parasites.
Many parasitic diseases now also occur in temperate industrial countries in
immunocompromised patients. The application of modern molecular techniques have
revolutionized the study of these diseases both in terms of diagnosis, vaccine
development and rational chemotherapy. In addition, it is increasingly clear
that many of the protozoa parasites are among the most deeply diverged
eukaryotic organisms and therefore represent model systems for the study of the
evolution of eukaryotes. Finally, it has become established that
symbiosis-parasitism was intimately associated with the origin of the eukaryotic
cell and that our cells represent the end product of eons of interactions
between different organisms and genomes.
Click here
for a list of events in the history of parasitology.
The images in this Chapter are taken from several sources:
Wellcome
Illustrated History of Tropical Diseases (Ed. F. Cox) Wellcome Trust Pub. Co.,
London (1996)
H. Scott (1939) A History of Tropical Medicine, Williams and
Wilkins Co. Baltimore.
Some questions to test your understanding of this
material:
1. What is parasitism? Is it unusual in biology?
2. Who started modern Parasitology? Why does Parasitology
not include the study of bacteria, fungi or viruses?
3. What was the major contribution of Leeuwenhoek? What
general principle is illustrated by Leewenhoek's work?
4. Why are Leishmania amastigotes often called
"Leishman-Donovan bodies"?
5. Did Ross discover that mosquitoes cause malaria? What
did he discover?
6. Who first realized that an insect can be the vector for
a parasitic disease?
7. What was unusual about Chagas's discoveries?
8. What was the major contribution of William Trager to
Parasitology?

.jpg)

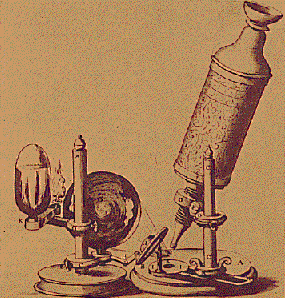













 Ross was born in India and went to Medical School in London. He
joined the Madras Medical Service and became interested in malaria. Ross showed
in 1895 that the parasites Laveran had seen underwent exflagellation in
Anopheles mosquitoes. In 1897 he tried breeding mosquitoes in the lab and fed
them on a patient with malaria. On August 20 he saw pigmented oocysts in the
wall of the stomach of one of these insects which had been fed 4 days earlier.
Ross then turned to the study of bird malaria. Several Italian scientists
carried on the study of human malaria. Ross unfortunately had a long feud with an Italian scientist, Grassi, on who
actually discovered the life cycle of Plasmodium. This, unfortunately, is also
the human side of science.
Ross was born in India and went to Medical School in London. He
joined the Madras Medical Service and became interested in malaria. Ross showed
in 1895 that the parasites Laveran had seen underwent exflagellation in
Anopheles mosquitoes. In 1897 he tried breeding mosquitoes in the lab and fed
them on a patient with malaria. On August 20 he saw pigmented oocysts in the
wall of the stomach of one of these insects which had been fed 4 days earlier.
Ross then turned to the study of bird malaria. Several Italian scientists
carried on the study of human malaria. Ross unfortunately had a long feud with an Italian scientist, Grassi, on who
actually discovered the life cycle of Plasmodium. This, unfortunately, is also
the human side of science. History of Parasitology, E.S. Livingston Ltd.,
London (1965)
History of Parasitology, E.S. Livingston Ltd.,
London (1965) 




