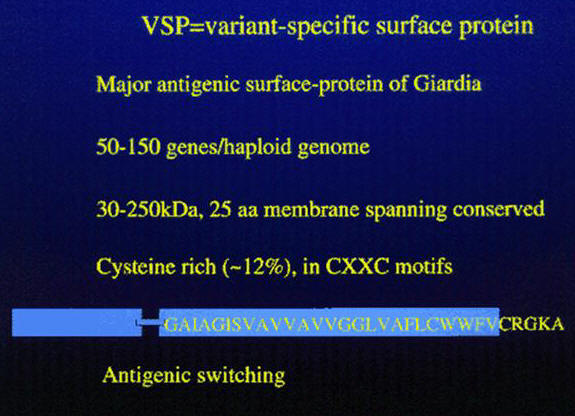
Antigenic Variation in Giardia
Giardia intestinalis undergoes surface antigenic variation, and the variant surface proteins (VSPs) form a coat 18nm thick, that covers the entire parasite including the flagella. In in vitro cultures the VSPs are shed into the medium. Antigenic variation occurs spontaneously both in vitro and in vivo, with the rates varying from 10-3 to 10-4. VSPs vary in molecular size (from 50kDa to 200kDa), are cysteine-rich containing 11-12% cysteine, which appears as CXXC motifs, but without free SH moieties, and these surface antigens have a conserved carboxy terminal region of 38 amino acids, which except for the last 5 amino acids (CRGKA) is hydrophobic; it is predicted that the hydrophobic tail serves as a transmembrane domain and the terminal CRGKA is in the cytosol. The VSPs also contain a conserved GGCY motif and one or more zinc finger domains. The amino terminal portion of the VSPs is the most variable and is also the portion of the molecule between the parasite and the its environment.
Surface switching of VSGs has been elegantly demonstrated using fluorescent activated cell sorting using monoclonal antibodies specific for two VSPs. Two VSPs were detected on the surface of single trophozoites and dual expression persisted for up to 30hr. In addition, it has been claimed that encystation and antigenic variation are mechanistically related processes.
The incubation of Giardia trophozoites with sera from infected individuals and with monoclonal antibodies to different VSGs resulted in a complement-dependent lysis of the parasites and at high antibody concentrations there is immediate immobilization and detachment and aggregation of trophozoites. In vivo the diversification of VSP expression occurs at about the time antibodies appear, and this is followed by continued diversity and preferential selection. Although such studies suggest that antigenic variation is a mechanism developed to escape the immune response of the host this need not be the sole biological function. For example, some free-living protozoans e.g. Paramecium and Tetrahymena, also undergo antigenic variation in response to environmental changes. And, since VSP expression changes occur in vivo even in the absence of an adaptive immune response the role of antigenic variation in Giardia must be more complex than simply immune evasion. Indeed, selection of antigenic type probably involves both antibody, principally IgA (which is secreted into the intestine), and non-antibody mechanisms such as the ability of the trophozoite to resist (via the disulfide-bonded cysteines of the VSP) host intestinal proteases.
About 2.5% of the Giardia genome is represented by VSP genes, and it is estimated there are 50-100 different VSPs. The first VSP gene, named TSA417, was cloned and expressed in 1990. The VSP genes are not near telomeres so that control of transcription does not appear to involve duplicative transposition to an expression-linked site near the telomere as occurs in Trypanosoma.
The DNA and amino acid sequence of TSA417 is shown below.
To study the fate of the TSA417 antigen during switching immunochemical procedures were used: The gene encoding TSA417 was cloned, expressed in bacteria, purified and rabbit antibodies to it prepared. The trophozoites of G. intestinalis were fixed and stained with the antiserum.Note that prior to encystation >85% of the cells are positive for TSA417 but 48 hr after encystation all but two cells express TSA417, indicating a switching of antigens during encystation/excystation.
From Svard et al. Molecular Microbiology 30 (5): 979-989.1998).
Prevalence of the TSA417 epitope on intact G.
lamblia trophozoites before encystation (A) and 48![]() h
after (B) excystation. Glutaraldehyde-fixed,
non-permeabilized G. lamblia WB clone C6
trophozoites were examined by immunocytochemistry
using polyclonal antirecombinant TSA 417 antiserum
and protein A-horseradish peroxidase.
Magnification 600.
h
after (B) excystation. Glutaraldehyde-fixed,
non-permeabilized G. lamblia WB clone C6
trophozoites were examined by immunocytochemistry
using polyclonal antirecombinant TSA 417 antiserum
and protein A-horseradish peroxidase.
Magnification 600.
Changes in VSP expression i.e. switching during excystation was also determined by immunoblotting
In this case a cellular extract of trophozoites at various times after excystation treatment (20 min, 90 min, 1 day, 3 days and 6 days) was prepared, size fractionated on SDS-PAGE, the proteins transferred to nitrocellulose, and the antigens on the nitrocellulose detected by reaction with rabbit antibody to TSA417.
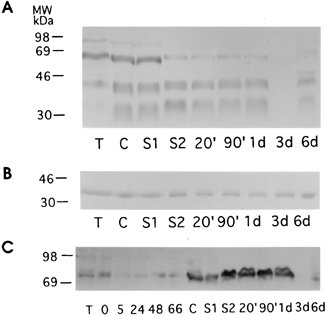
A. TSA417. B. Taglin
(control). C. VSP-rB2.
From Svat et al.
Northern Blot Analysis
To determine whether the mRNA that encodes the protein of interest (e.g. TSA 417) is expressed in specific cells or at specific times Northern blot analysis was carried out. To carry out Northern blot analysis total RNA was prepared, the RNA was size separated using agar gel electrophoresis, and the RNA from the gel was transferred to a nylon membrane and the RNA of interest was detected by hybridization with the gene (in this case a TSA417-specific oligonucleotide) that encodes that RNA.
TSA417 mRNA is
undetectable 90' after encystation. VSP cons
non-specifically detects other VSPs. Shows that
new VSP mRNAs are expressed. PDI-1 is a
constitutively expressed control RNA.
From these experiments it can be concluded: 1. during excystation/encystation expression of the “old” VSP on the trophozoite surface is turned off, 2. “new” VSP is expressed on the newly emerged trophozoite after encystation/.excystation, and 3. VSP expression is controlled at the level of transcription.
An important question