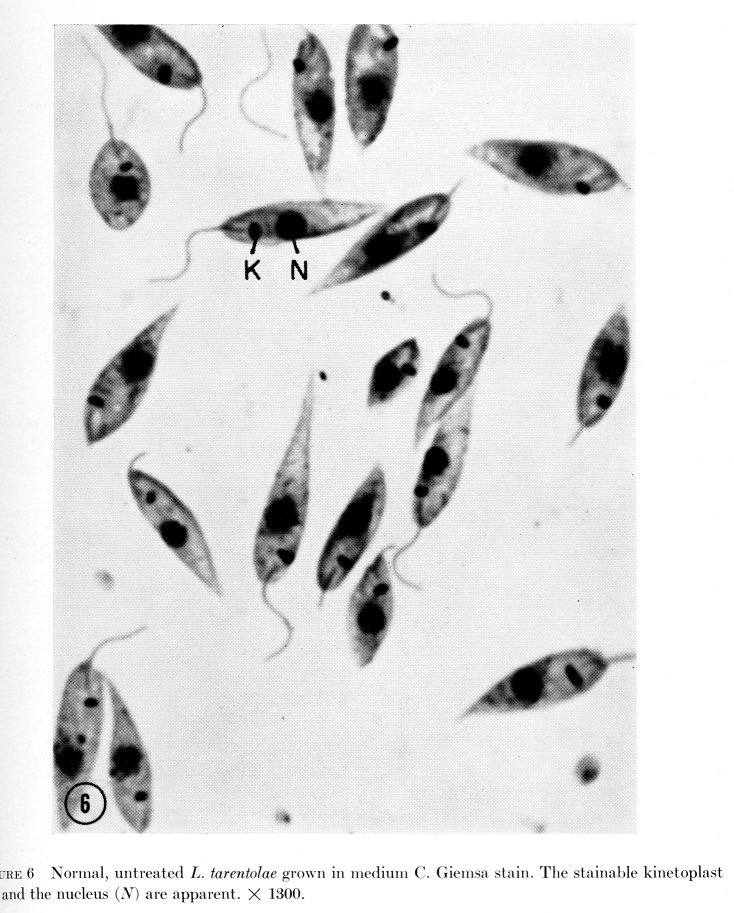
In the early 1900's F. Werbitzki, a student of Paul Erlich,was attempting to cure trypanosomiasis in animals by treatment with a variety of acridine dyes such as pyronine, oxazine and rosanaline. He discovered that a strain of trypanosomes that had become resistant to the dye had lost the kinetoplast. We now know that the kinetoplast was actually the network of kDNA within the mitochondrion. He called these "blepharoblastlose" since this organelle was then called the blepharoblast. He was able to obtain a 100% akinetoplastic line by repeated passage with oxazine treatment. These parasites were infective and had normal motility.
After the existence of mitochondrial DNA in yeast and mammalian cells was discovered in the 1960's, Ephrussi and Slonimski found that there was a very high frequency of mutation of the mtDNA in Baker's yeast to produce petites, some of which had mutated mtDAN and some of which had no mtDNA (r- cells). This phenomenon appears similar to that in trypanosomes since it is also induced by dye treatment.
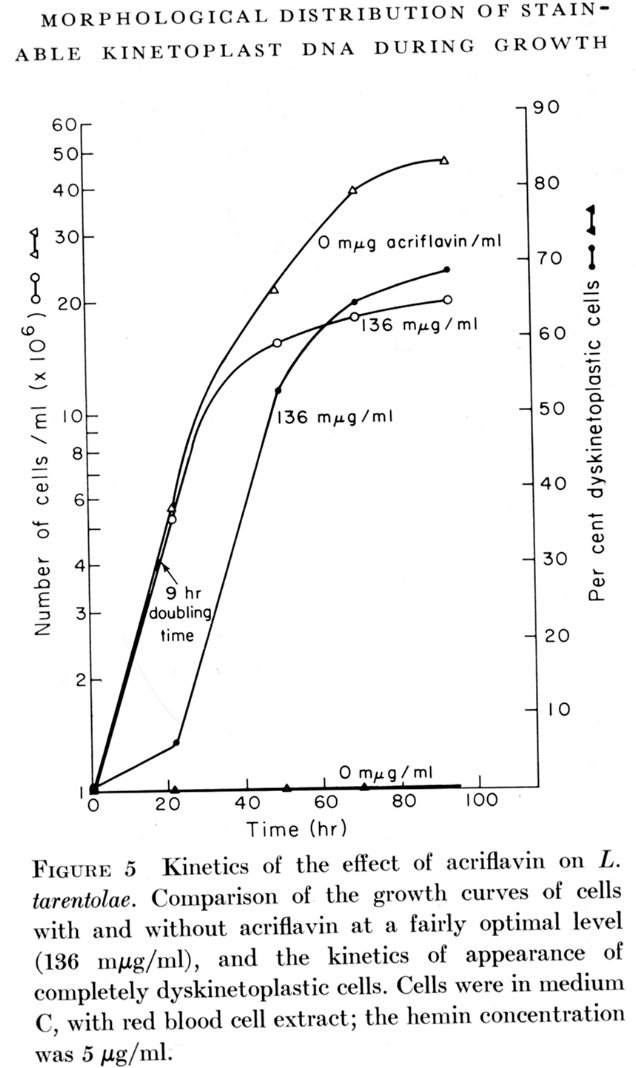
Steinert showed in 1967 that the intercalating dye, ethidium bromide, produced a loss of kDNA in cultured trypanosomatids. This phenomenon was analyzed in detail in 1968 using Leishmania tarentolae cells in culture. Low levels of acriflavin produced high levels of dyskinetoplastic cells which were not viable in culture.


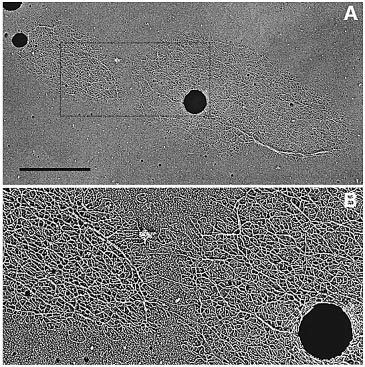
The amount of kinetoplast DNA appeared to decrease and then there was a final all-and-none kinetoplast division resulting in one daughter dyskinetoplastic cell and one with a small kinetoplast, as shown below:
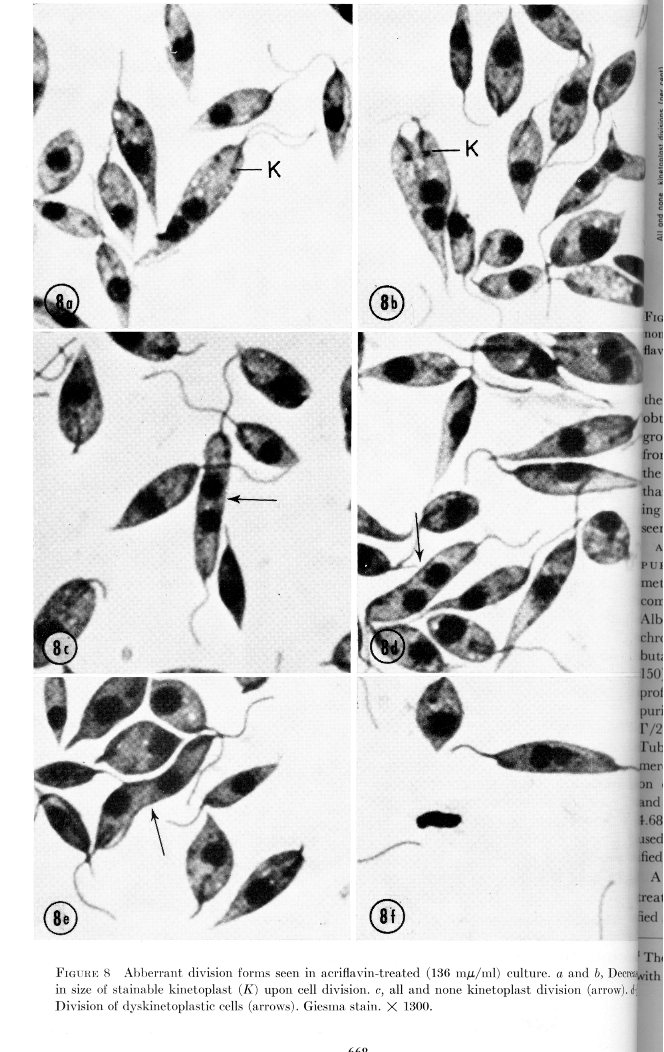
This was correlated with a specific kinetoplast localization of the dye as shown below:
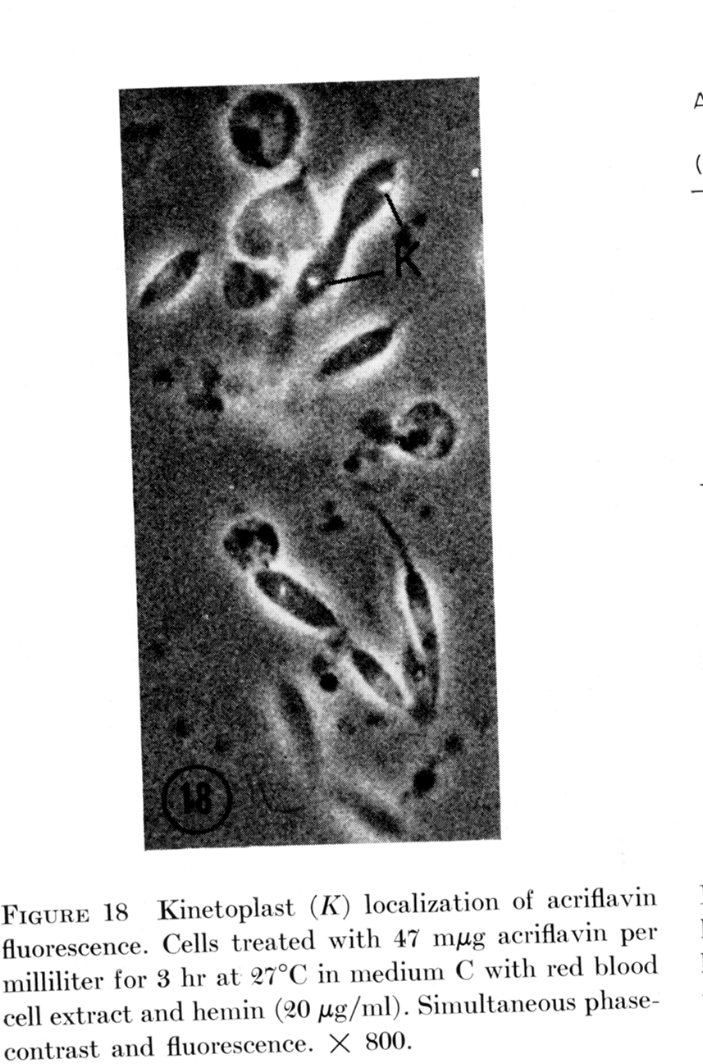
It was concluded that the dye was taken up specifically by the kinetoplast-mitochondrion, and the intercalation of the dye selectively inhibited kDNA replication and segregation.
(From Simpson, 1968. J. Cell Biol. 37, 660)
A recent elegant paper has shown in the case of procyclic T. brucei that conditional RNAi down-regulation of the mitochondrial Topoisomerase II caused a progressive shrinking of the kDNA network by asymmetrical segregation at cell division. Cell viability was affected and changes in the gRNA-encoding minicircle repertoire were observed. These results are remarkably similar to those obtained in the earlier Leishmania studies using acriflavin. Figures below are from this paper.
|
|
|
 kDNA network undergoing asymmetrical division. |
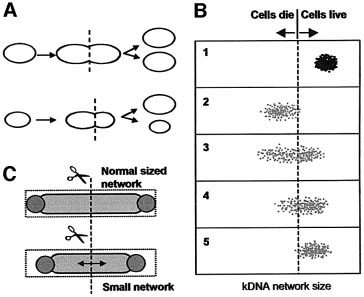 Models for asymmetrical cleavage of kDNA network. |
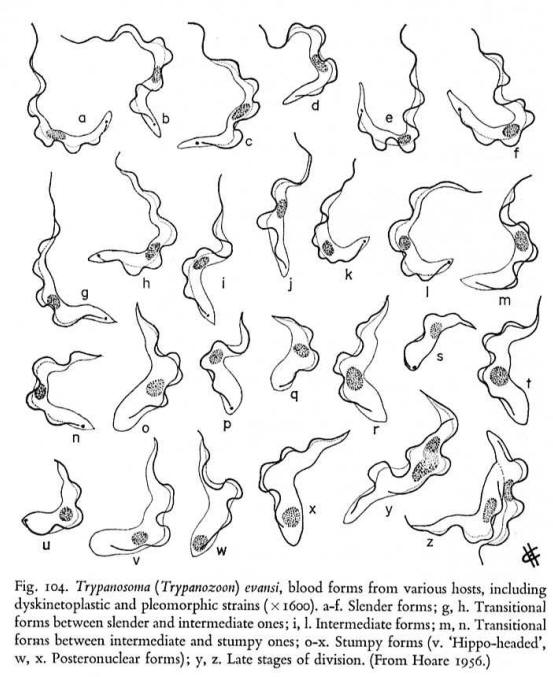
Dyskinetoplastic African Trypanosomes
Complete dyskinetoplastidy and modifications in the kDNA occurs in nature in some strains of T. evansi ,T. equiperdum and T. equinum. These parasites cause disease in animals. T. equiperdum is a venereal disease and T. evansi and T. equinum are transmitted by flies acting as "flying syringes". It is not a coincidence that these parasites lack an intermediate host and are transmitted mechanically. It appears that a respiratory competent mitochondrion is required for life in the insect vector and can be dispensed with in the bloodstream stage where electrons are taken to a CN-insensitive alternative oxidase for reoxidation of NADH. However recent work has shown that down regulation of RNA editing in bloodstream T. brucei by RNAi of an RNA ligase is lethal, bringing into question this old assumption.
One strain of T. equiperdum has deletions and rearrangements in the maxicircle genome and an apparent single major sequence class of minicircles. Dyskinetoplastic T. equiperdum were produced in the rat by continuous growth with ethidium bromide or acriflavin. And after 7 passages in the absence of the dye the cells were viable and still contained no kDNA. T. evansi does not appear to have maxicircle DNA at all and also has a major minicircle sequence class. Some naturally occurring strains of T. evansi have no detectable kDNA.
Note the presence of naturally occurring dyskinetoplastic forms.
Computer Modeling of Loss of kDNA Minicircle Sequences Classes in Culture
Computer modeling has shown that loss of specific minicircle sequence classes can occur if minicircles are segregated randomly at division of the network.
Savill and Higgs modeled the frequencies of minicircles in the network, provided several assumptions were made. These included the assumption that segregation of minicircles at division of the network is completely random, and that loss of certain minicircle sequence classes encoding gRNAs for editing is lethal and loss of other classes is non-lethal. They also assumed a fixed number of minicircles per network.
This model was used to compare to experimental results obtained by Simpson et al. 2000 in changes in minicircle class frequencies during long term culture of L. tarentolae in the laboratory.
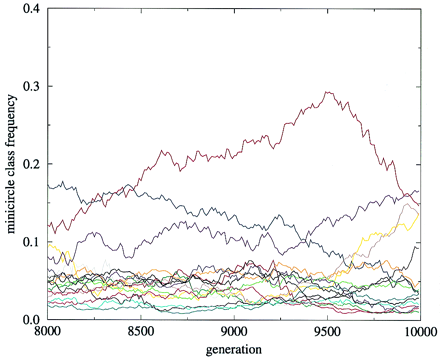 The
average frequencies of 17 minicircle classes
undergoing random segregation over
2,000 generations. Random segregation causes
fluctuations in the frequencies, giving rise to
the experimentally observed distribution of many
classes having low frequency and few classes
having high frequency. Initially at generation
zero, every class in every cell has 170 copies.
There are 1,000 cells that have maximum network
sizes of 12,000 minicircles.
The
average frequencies of 17 minicircle classes
undergoing random segregation over
2,000 generations. Random segregation causes
fluctuations in the frequencies, giving rise to
the experimentally observed distribution of many
classes having low frequency and few classes
having high frequency. Initially at generation
zero, every class in every cell has 170 copies.
There are 1,000 cells that have maximum network
sizes of 12,000 minicircles.
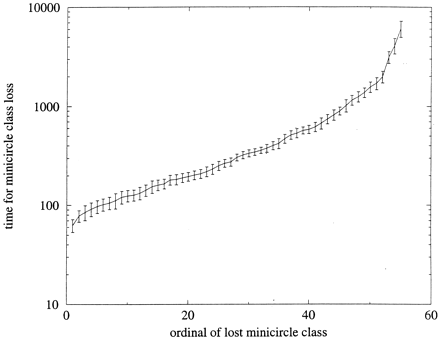
The number of generations for consecutive unnecessary minicircle classes to be lost. The last few classes take many thousands of generations to be lost. The simulations are initially run for 2,000 generations with all classes being necessary. This is to lose the artificial initial conditions. Then, 55 classes become unnecessary, i.e., if their frequencies reach zero in a cell, the cell is still viable. The loss time was averaged over 10 simulations; error bars show ± 2 SEM.
As
unnecessary classes are lost, one unnecessary
class must become highly abundant to maintain the
size of the kinetoplast, i.e., 10,000 minicircles.
This is because the 15 necessary classes are
restricted to a maximum of only 200 copies.
Initially all classes have a copy number of
170, and 55 classes are unnecessary and 15 are
necessary. The red line shows the cumulative
frequency of all unnecessary classes, the black
line the cumulative frequency of all necessary
classes, and the green line the frequency of the
most abundant class at any given time.
All of these predictions fit the observed plasticity of minicircle sequence classes in the old UC lab strain of L. tarentolae in culture. Figures from Simpson et al. 2000
Questions:
1. Cells lacking detectable kDNA or having smaller amounts of kDNA can be obtained experimentally. When is this lethal and when is it not lethal?
2. How has the loss of kDNA occurred in nature and how is this related to the type of vector utilized by the parasite?