| |
Prepared in collaboration with Noriyuki Murakami
Outline of Sections
 |
Summary of Basic Science and Clinical Information for African Trypanosomiasis (PDF)
(Chapter 6 from: Parasitic Diseases 5th Ed.) |
Section 1 |
|
Section 2 |
|
Section 3 |
|
Section 4 |
|
4.1 |
|
4.1.1 |
|
4.1.2 |
|
4.1.3 |
|
4.2 |
|
4.2.1 |
|
4.2.2 |
|
Section 5 |
|
5.1 |
|
5.2 |
|
5.3 |
|
5.4 |
|
Section 6 |
|
6.1 |
|
6.2 |
|
6.3 |
|
6.4 |
|
6.5 |
|
Section 7 |
|
Section 8 |
|
12/24/05 |
|
Sleeping sickness, also known as Human African Trypanosomiasis (HAT), is caused by Trypanosoma brucei rhodensiense in Eastern Africa and Trypanosoma brucei gambiense
in Western Africa . Both protozoan species are morphologically
indistinguishable, but have drastically different epidemiological
features. Several species of hematophagous glossina, commonly known as
tsetse flies, are the vectors of these related diseases, and are
responsible for cyclical transmission of the parasitic protozoan between
numerous vertebrate hosts. Both forms of sleeping sickness affect the
central nervous system. The term “sleeping sickness” is derived from the
West African form of trypanosomiasis, primarily because invasion of the
cerebrospinal fluid and brain after infection of the blood is often
delayed, resulting in symptoms of extreme fatigue that can last for
several years before the severe phase of the disease sets in; toxemia,
coma and death. In contrast, the typical East African form of
trypanosomiasis is characterized by rapid and acute development of the
disease, and untreated patients can die within weeks or months of
infection.
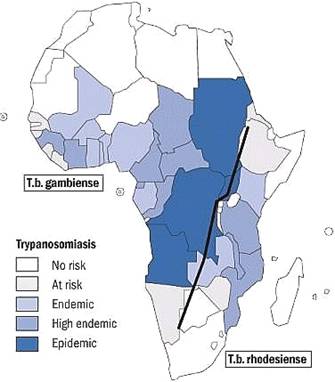 |
East & West African Sleeping Sickness
(Map provided by WHO) |
Although epidemics of sleeping
sickness were more rampant in the past, the most recent WHO estimates
put 60 million people at risk of HAT today with approximately 500,000
people currently with infections. The disease is discontinuously spread
over 9 million square kilometers and affects populations across 37
sub-Saharan countries. Animal trypanosomiasis, caused by a wider number
of trypanosome species and carried with higher prevalence by a greater
number of glossina species, is invariably the greater epidemic across
the African continent with dire economic consequences. In general,
trypanosome infections that threaten livestock are over 100 to 150-fold
higher in G. morsitans than the trypanosome infections that
cause human trypanosomiasis (Jordan 1976). Historically, the impact of
animal trypanosomiasis were so profound that it influenced the migration
routes of cattle-owning tribes into the continent who were forced to
avoid the G. morsitans “fly-belts” (Ford 1960), as well as the movements
of early European and Arab settlers into the continent who depended on
horses and oxen (McKelvey 1973).
Of the 31 species of glossina in
the African continent, eleven are important for transmitting the
infection to humans. Today, most efforts to reduce transmission of the
disease to humans and other vertebrate reservoirs focus on the control
of only these species. Clinical treatment of both early and late onset
sleeping sickness is limited and far from up-to-date, and thus cannot be
relied upon for controlling the spread of the infection during times of
epidemics. Therefore, understanding the ecological factors that
determine patterns of transmission to people, and those that play a role
in the re-emergence of the disease is vital to the design of new
effective programs to reduce the burden of disease in human populations.
This web site emphasizes the ecology of the tsetse vectors and also
documents some of the more useful control strategies to limit their
populations.
The earliest recorded account of sleeping sickness comes from upper Niger during the 14th
century in the historical writings of Ibn Khaldoun, who wrote about the
disease in his account of the history of North Africa . The next report
came from Guinea in 1734 (Atkins, 1978). In 1803, the diseases that
caused visible swollen lymph glands in West Africa came to be known as Winterbottom's sign,
after the description of the disease by Winterbottom. Such signs were
readily recognized by slave traders who avoided trading and buying
slaves who displayed those symptoms.
 |
Sir David Bruce |
The earliest detection of
trypanosomes in human blood was in 1902, when R.M. Forde discovered what
was then thought to be filiaria in the blood of a steamboat captain who
had traveled extensively along the River Gambia. Similar discoveries of
filiaria-like organisms in the blood were made by J.H. Cook in East
Africa, but confusion arose as to how filiaria worms could cause such
varying clinical symptoms. It was J.E. Dutton who, during a visit to
Gambia, first correctly identified the parasite as a trypanosome and
subsequently named it Trypanosoma gambiense . In
1902, A. Castellani observed the presence of trypanosomes in
cerebrospinal fluid taken from a sleeping sickness patient, but it
wasn't until 1903 that D. Bruce correctly recognized that trypanosomes
were the causative agents of sleeping sickness transmitted to humans by
tsetse flies, and that “trypanosome fever” and “sleeping sickness” -
both thought to be different diseases at the time - were in fact the
same.
Morphologically indistinguishable from the West African species as well as the animal infecting species Trypanosoma brucei brucei, Trypanosoma brucei rhodensiense was first discovered in Zambia by J.W.W. Stephens and H.B. Fantham in 1910. By 1926, T.b. rhodensiense
could be found along the fly-belt between Tabora and Kigoma, Tanzania .
The difficulties in identifying this virulent form of sleeping sickness
lead to uncertainties today regarding the evolution and progression of T.b. rhodensiense through the continent, although it is generally agreed upon that it originated from the West African form.
The earliest recorded major
epidemics of sleeping sickness took place in Uganda and Congo between
1896 and 1908, where roughly 500,000 people were estimated to have died
in the Congo Basin, and approximately 300,000 died in Busoga, Uganda .
With the Rift Valley transecting the country, Uganda is in the
precarious position of having foci of both forms of diseases which
resulted in two other major epidemics of sleeping sickness - one in the
late 1940's and another in 1980. Throughout West Africa, smaller
epidemics of sleeping sickness rapidly spread from Senegal to Cameroon
during the 1920's, and died down by the late 1940's.
African trypanosomes are extracellular organisms, both in the mammalian and insect host. T. b. gambiense and T. b. rhodesiense are morphologically indistinguishable, measuring 25-40 µ m
in length. Infection in the human host begins when the infective stage,
known as the metacyclic stage, is injected intradermally by the tsetse
fly. The organisms rapidly transform into blood-stage trypomastigotes
(long, slender forms), and divide by binary fission in the interstitial
spaces at the site of the bite wound. The buildup of metabolic wastes
and cell debris leads to the formation of a chancre  . .
Trypanosomes have a single specialized mitochondrion called a kinetoplast mitochondrion. One
of its unusual features is that all of the DNA of the mitochondrion,
which can be up to 25% of the total cell DNA, is localized in the
kinetoplast, adjacent to the flagellar pocket. Kinetoplast DNA or kDNA
exists in two forms: mini-circles and maxi-circles. Mini-circle DNA
encodes guide RNAs that direct extensive editing of RNA transcripts
post-transcriptionally. Maxi-circle DNA contains sequences that, when
edited, direct translation of typically mitochondrially-encoded
proteins.
In the vertebrate host,
trypanosomes depend entirely upon glucose for energy and are highly
aerobic, despite the fact that the kinetoplast-mitochondrion completely
lacks cytochromes. Instead, mitochondrial oxygen consumption is based on
an alternative oxidase that does not produce ATP. When in the insect
vector, the parasite develops a conventional cytochrome chain and TCA
cycle.
 |
The surface of the trypanosome has numerous membrane-associated transport proteins for
obtaining nucleic acid bases, glucose, and other small molecular weight
nutrients. None of these proteins react well with antibodies, because
although they lie in exposed regions of membrane, they are shielded by
allosteric interference provided by the variant surface glycoprotein
(VSG) coat proteins. This flagellated stage enters the bloodstream
through the lymphatics and divides further, producing a patent
parasitemia. The number of parasites in the blood is generally so low
that diagnosis by microscopic examination is often negative. At some
point, trypanosomes enter the central nervous system, with serious
pathological consequences for humans. Some parasites transform into the
non-dividing short, stumpy form  , which has a biochemistry similar to those of the long, slender form and the form found in the insect vector. , which has a biochemistry similar to those of the long, slender form and the form found in the insect vector.
The tsetse fly becomes infected
by ingesting a blood meal from an infected host. These short, stumpy
forms are pre-adapted to the vector, having a well developed
mitochondrion with a partial TCA cycle. In the insect vector, the
trypanosomes develop into procyclic trypomastigotes  in the midgut of the fly, and continue to divide for approximately 10
days. Here they gain a fully functional cytochrome system and TCA cycle.
When the division cycles are completed, the organisms migrate to the
salivary glands, and transform into epimastigotes. These forms, in turn,
divide and transform further into metacyclic trypanosomes, the
infective stage for humans and reservoir hosts. The cycle in the insect
takes 25-50 days, depending upon the species of the fly, the strain of
the trypanosome, and the ambient temperature. If tsetse flies ingest
more than one strain of trypanosome, there is the possibility of genetic
exchange between the two strains, generating an increase in genetic
diversity in an organism that may not have a sexual cycle.
in the midgut of the fly, and continue to divide for approximately 10
days. Here they gain a fully functional cytochrome system and TCA cycle.
When the division cycles are completed, the organisms migrate to the
salivary glands, and transform into epimastigotes. These forms, in turn,
divide and transform further into metacyclic trypanosomes, the
infective stage for humans and reservoir hosts. The cycle in the insect
takes 25-50 days, depending upon the species of the fly, the strain of
the trypanosome, and the ambient temperature. If tsetse flies ingest
more than one strain of trypanosome, there is the possibility of genetic
exchange between the two strains, generating an increase in genetic
diversity in an organism that may not have a sexual cycle.
Flies can remain infected for
life (2-3 months). Tsetse flies inject over 40,000 metacyclic
trypanosomes when they take a blood meal. The minimum infective dose for
most hosts is 300-500 organisms, although experimental animals have
been infected with a single organism. Infection can also be acquired by
eating raw meat from an infected animal. In East Africa, this mode of transmission may be important in maintaining the cycle in some reservoir hosts.
4 |
Medical Ecology of Sleeping Sickness
Part I: The Vector |
|
4.1 |
Classification and Distribution |
|
 |
| |
There are 31 species and subspecies of tsetse flies under the genus Glossina, family Glossinidae, and order Diptera.
Tsetse flies are largely classified into three subgenera based on
morphological differences in the structure of the genitalia: Morsitans (Glossina), Palpalis (Nemorhina), and Fusca (Austenina)
groups. Although the tsetse flies can be found over some 9 million
squared kilometers of the African continent, presence of glossina
populations throughout the continent are far from continuous. In
general, the Sahara and Somali Deserts limit the populations in the
north, extending across the entire continent from Senegal in the west to
southern Somalia in the east. Tsetse populations are denser in West and
Central Africa, and are found more sporadically to the East and down to
the borders of the Kalahari and Namibian Deserts in Southern Africa .
Although tsetse fly habitats may vary considerably, climate and altitude
- through their direct effects on vegetation, rainfall, and temperature
- are still the primary determinants for proliferation. Unlike other
insects, there are no seasonal interruptions in the life cycles of
tsetse flies. However both adult longevity and puparial duration are
related to temperature, and a significant seasonal decline in tsetse
populations is normal, particularly in savannah habitats during the dry
season. The 3 groups of tsetse flies are generally adapted to different
habitats and ecozones.
Brief summary of ecological zones in Africa (adapted from Jahnke, 1982)
| Characteristics |
Ecological Zones |
| |
Arid |
Semi-arid |
Sub-humid |
Humid |
Highlands |
Area (1000km2) |
8327 |
4050 |
4858 |
4137 |
990 |
Rainfall (mm) |
<500 |
500-1000 |
1000-1500 |
>1500 |
Variable |
Moisture Index |
36 |
20 |
0-20 |
0 |
Variable |
Growing Days |
<90 |
90-180 |
180-240 |
>240 |
Variable |
Area of Tsetse (1000km2) |
438 |
2036 |
3298 |
3741 |
195 |
Area of Tsetse % |
4.2 |
50.3 |
68.2 |
89.7 |
1.9 |
Predominant Tsetse Group |
Morsitans |
Morsitas
Palpalis |
Palpalis
Fusca |
Fusca
Palpalis |
None |
4.1.1 |
Habitat and Distribution of the Morsitans Group |
|
There are seven species that fall
into the morsitans group. All are potential vectors of both human and
animal trypanosomiasis. The three Glossina morsitans
subspecies are exceptionally good vectors of trypanosomes. All species
within this group inhabit the savanna woodlands that surround the two
major blocks of lowland rain forests in Africa . The distributions of
tsetse flies in this group closely follow the distributions of wild
animals and water sources. In the wetter areas the flies are observed to
roam more widely over the woodland, but in drier areas their movements
are restricted to mesophytic vegetation of the watercourses.
In Eastern and Southern Africa where Glossina morsitans morsitans is the primary vector for human and animal trypanosomiasis, the "miombo" woodlands (Brachystegia-Julbernardia) that extend from Mozambique to Tanzania, as well as the "mopane" woodlands (Colophospermum mopane) in Zambia and Zimbabwe are the typical habitats. The other subspecies Glossina morsitans centralis
dominate northwards from Botswana and Angola into Southern Uganda,
closer inland towards the lowland forests but also occurring in miombo
vegetation. Glossina morsitans submorsitans have an east to
west distribution from Ethiopia to Senegal in ‘doka' woodlands where the
vegetation is dominated by Isoberlinia species, and can be sporadically
found to occur in the southern Guinea savanna vegetation zone as well
in the drier Sudan zone.
Glossina swynnertoni is restricted to a small area between northern Tanzania (Serengeti) and Southern Kenya (Masai Mara) where the Acacia-Commiphora vegetation can be found, along with an abundance of wild life. Glossina longipalpis and Glossina pallidipes
both have a much wider range of possible habitats displaying
versatility by existing in different vegetation types. Glossina
longipalpis occurs mainly in the narrow savannah belt just north of the
rain forest in West Africa, from Guinea to Cameroon . Much of the
savannah is derived from human destruction of the climax forest
vegetation, and as a result is spreading southwards. The highly mobile
Glossina pallidipes occurs in East Africa from Mozambique to Ethiopia
over a relatively wide range of climatic and vegetation conditions.
Finally, Glossina austeni occupy secondary scrub, thicket and
islands of forest along the East African coast from Mozambique to
Somalia. However, its distribution is discontinuous, rarely being found
at altitudes over 200 meters or more than 250 km inland from the coast.
4.1.2 |
Habitat and Distribution of the Palpalis Group |
|
see distribution of palpalis group in Africa
Of the nine species in the palpalis subgenera, the five palpalis and fuscipes subspecies are vectors of both human and animal trypanosomiasis. Although flies in this group are continuously found in the lowland rainforests,
some are known to extend out to the savannah regions particularly along
rivers and streams. The habitat of the palpalis flies occur mainly in
the drainage systems leading to the Atlantic or the Mediterranean Ocean,
extending from the wet mangrove
and rain forests along the coastal regions of West Africa to the drier
savannah areas just north of the rain forests. The flies in the palpalis
group are less tolerant to the wide range of climatic conditions of the
savannah belt, and are therefore restricted to the ecoclimate of the
watercourses from where they derive their label as the ‘riverine
species'. Many of the palpalis species, such as the Glossina palpalis palpalis
in Côte d'Ivoire, prefer peri-domestic conditions and have been
observed to maintain close association with villages (Baldry, 1980).
Similarly, it is thought that the advancement of Glossina tachinoides
into Eastern Côte d'Ivoire and Togo have been attributed to the intense
agricultural development and the rapid human population growth around
the plantations (Hendrickx and Napala, 1997). In general, most of the
flies in this group are less suited to desiccating conditions, and
therefore survive in thick riverine forests with enough shelter from
winds and heat. This is especially the case for the three fuscipes
subspecies which are confined to hygrophytic habitats, rarely far from
open water lacustrine or riverine habitats. Glossina tachinoides,
although typically a riverine species, were found in northern Nigeria
to extend into human-inhabited savanna woodlands during the wet season,
also displaying strong adaptations to peridomestic habitats (Kuzoe et
al., 1985).
4.1.3 |
Habitat and Distribution of the Fusca Group |
|
see distribution of fusca group in Africa
With the exception of Glossina brevipalpis and Glossina longipennis,
all the tsetse flies in the fusca group are found in West African
forests. None of the species in the fusca group are vectors of human
trypanosomiasis, however both Glossina fusca and Glossina medicorum are efficient vectors of trypanosomes to livestock (mainly Trypanosoma vivax),
causing considerable economic burden. Distributions of the fusca group
tsetse depend primarily on forest vegetation and climatic factors. With
the exception of G. longipennis, most fusca group species inhabit moist,
evergreen habitats either in riverine forests within savannas (such as Glossina medicorum) or in dense and wet rain forests (Glossina tabaniformis and Glossina nigrofusca).
In stark contrast to the rest, the G. longipennis species lives in one
of the driest habitats inhabited by tsetse flies. Due to its pupal
adaptation to dry conditions, its primary habitat - consisting of dry
deciduous acacia bush – are discontinuously spread throughout East
Africa (Glasgow, 1963).
4.2 |
Susceptibility Factors |
|
The intrinsic vectorial capacity
of a tsetse fly refers to the intrinsic capability of a fly to develop a
metacyclic infection (Le Ray, 1989). In general, infection rates of the
three salivarian trypanosome subgenera (Dutonella, Nannomonas, and
Trypanozoon) are usually low in populations of tsetse, with infections
rates determined by the parasite, the host, the vector, and the
environment (Jordan, 1974). Trypanosoma infection rates in tsetse flies
vary greatly from species to species, with T. vivax ranking the highest and T. brucei species ranking the lowest.
Factors Influencing Trypanosome Infection Rates in Tsetse
(adapted from Jordan 1974 and Molyneux 1980)
| Endogenous Factors (Vector) |
Parasite Factors |
| Tsetse species |
Parasite numbers available to tsetse |
| Sex |
Parasite species and its infectivity to tsetse |
| Age at infective feed |
Subspecies/strain |
| Age structure of tsetse population |
|
| Genetic differences (variations within species) |
|
| Behavior (host preferences) |
|
Concurrent infections (virus, bacteria, fungi) |
|
Interactions between lectins and Rickettsia-like organisms |
|
Physiological and biochemical state |
|
| |
|
| Ecological Factors (Environment) |
Host Factors |
| Climatic factors |
Susceptibility |
| Availability of infected hosts |
Immune state of host |
| Hosts available for subsequent feed |
Behavior and attractiveness to tsetse |
Tsetse fly species differ in
their ability to develop infections, as discussed earlier. Female tsetse
flies usually have higher infection rates than males, partially because
females live longer than males and therefore have a higher probability
of infection. However is has not yet been determined whether the sex of a
fly influences the infection rate (Kazadi 1991, Mihok, 1992). Within
species it is found that infection rates vary greatly depending on
individual host factors. It has also been shown that susceptibility of
flies to infection with T. brucei is also due to a maternally inherited
characteristic, associated with the presence of intracellular
rickettsia-like organisms (RLOs). Tsetse flies carrying RLOs in the
midgut were found to be six times more likely to be infected with
trypanosomes than those without (Maudlin et al., 1990). Within any given
species, individuals and sexes of the same species respond differently
to infection suggesting some involvement of the genetic differences
(Jordan, 1974).
4.2.1 |
Behavioral Ecology of the Vector: Mating |
|

The mating behavior of tsetse
flies have received much attention because of the development of the
Sterile Insect Technique (SIT) for tsetse fly control. The existence of
tsetse flies at low densities in certain areas (as low as 40 per km2)
suggest highly specific mating mechanisms involving visual and
olfactory responses (Glasgow, 1963). Female tsetse flies only need to
mate once in their lifetime, but multiple matings have been known to
occur occasionally (Jaenson, 1980). Cross-mating is possible in areas
where habitats of different species overlap, however male hybrids are
infertile (Jackson, 1950). Mating of female is mostly confined to early
life with mean duration of mating declining by age. Most female flies
are successfully inseminated even at very low population densities,
usually during their first blood meal right after emergence from the
pupal stage (Teesdale, 1940).
4.2.2 |
Behavioral Ecology of the Vector: Host Feeding |
|
Tsetse flies use visual and
olfactory characteristics to recognize potential hosts before initiating
host-oriented responses. There are a series of behavioral responses
involved in the process of obtaining a bloodmeal. Host-seeking behaviors
are influenced by endogenous and exogenous factors. Endogenous factors
include circadian rhythm of activity level of starvation, age, sex and
pregnancy status of the fly (Brady, 1972). Exogenous factors include
temperature, vapor pressure, visual and olfactory stimuli, and
mechanical stimulation (Huyton and Brady, 1975). There are four stages
of host-locating behavior as described by Wilemse and Takken (1994):
Ranging
Flying in search of a host in the absence of an external cue.
Activation
Change in behavior caused by perception of external stimulus
Orientation
Upwind anemotaxis in response to complex chemical and visual stimuli directing the insect to the host
Landing
Generally, the tsetse fly will
detect an odour plume upwind until it visually recognizes the host.
After landing on the host, heat stimulation cause a probing and feeding
response. It was found that feeding activity for the morsitans group
tsetse were highest during the early morning and the late afternoon due a
combination of both external temperatures (20%) and circadian rhythm
(80%) (Brady and Crump, 1978).
It is thought that tsetse flies
originally fed on reptiles living in forests and later became adapted to
feeding on mammals. Adaptations to feeding on warthogs are thought to
be one of the pathways by which tsetse flies entered the savanna
ecosystems, subsequently evolving as the separate morsitans subgenus
(Ford, 1970). Most of our knowledge about species specific host
preferences is derived from blood meal analyses of captured flies.
Summary of main hosts of tsetse determined from analysis of bloodmeals (adapted from Moloo, 1993)
Host |
Morsitans Group |
Palpalis Group |
Fusca Group |
Domestic animals including human |
Human |
3.6 |
13.00(3) |
0.46 |
Cattle |
3.56 |
4.2 |
2.38 |
Sheep and Goats |
0.26 |
0.4 |
0.2 |
Donkey |
0.02 |
0.05 |
0.02 |
Dogs |
0.15 |
0.26 |
0.02 |
Domestic Pigs |
0.03 |
14.00(2) |
0.17 |
|
|
|
|
|
Sylvatic hosts |
Bushpig |
5.60 |
2.20 |
28.0(1) |
Warthog |
31.60(1) |
1.65 |
0.37 |
 Total Suidae Total Suidae
|
45.36 |
18.50 |
33.1 |
|
|
|
|
Bushbuck |
10.18(2) |
25.12(1) |
11.2(2) |
Buffalo |
7.50(3) |
0.90 |
7.59(3) |
Kudu |
5.37 |
|
|
 Total Bovidae Total Bovidae
|
43.22 |
45.50 |
28.1 |
|
|
|
|
Monitor Lizard |
0.036 |
6.02 |
0.05 |
Hippopotamus |
|
4.04 |
15.84* |
Rhinocerus |
|
|
14.20* |
Numbers in parentheses show host ranking in order of importance for each subgenera.
* Ranking for anomalous species G. brevipalpis and G. longipennis
|
4.3 |
Life Cycle of the Vector |
|
Unlike most insect species
(r-strategists) that produce large quantities of eggs, fertilized female
tsetse flies (k-strategists) “give birth” to one larva. A typical
female tsetse fly will produce one full grown larva approximately every
9-10 days depending on temperature and humidity. A single egg will hatch
and develop to a third-stage larva in the uterus of the female fly,
where it is nurtured and supplied with nutrients. This reproductive
process is known as adenotrophic viviparity . This form of
reproduction ensures the higher degree of survival of each offspring,
but is also the reason why reproductive rates are considerably low in
tsetse fly populations. In laboratory colonies, a single adult female
can produce up to 12 offspring this way, however in the wild the number
is speculated to be lower (Leak, 1999). Larviposition takes place when
the third-instar larva is deposited onto a suitable site, usually soil
or sand depending on the species, and the larva burrows down to its
optimum depth to become a pupa. Usually an adult fly will emerge after
the puparial period which varies according to temperature but on average
is around 30 days at 24°C (Leak, 1999). Longevity of the adult fly
varies greatly according to seasonal factors. For the general tsetse
population to increase, it is critical that the average female lifespan
exceed 36 days. During optimal conditions, female flies can live as long
as 3 months, producing as much 10 offspring during her lifetime
(Jordan, 1986).
Many population control methods
in the past have been successful because tsetse populations are far more
vulnerable to disruptions in life cycles than insects that are
r-strategists, such as mosquitoes. When both larval and adult mortality
rates are artificially increased through control methods such as fly
traps, insecticide spraying, and sterile insect techniques, the
reduction in reproductive rate is profound.
5 |
Medical Ecology of Sleeping Sickness
Part II: The Human |
|
see WHO annually reported cases of sleeping sickness by African countries
Although epidemics as large as
the ones in Uganda at the turn of the century have not been repeated,
there is much concern over the re-emergence and increase in the number
of sleeping sickness cases being reported every year in Africa. In 1994,
there were an estimated 150,000 cases in Congo, with prevalences as
high as 70% in some villages (Cattand, 1994). Despite the WHO projection
of 60 million people at risk in Africa, only a fraction of the
population at risk is currently under surveillance, and relatively few
cases are accurately diagnosed annually (Knudsen and Slooff, 1992).
Although sleeping sickness was largely under control during the 1960s,
recent epidemics have been strongly associated with political and civil
unrest in West and Central Africa resulting in mass movement of
populations into areas formerly uninhabited by humans.
5.1 |
Epidemiology of West African Sleeping Sickness |
|
West African sleeping sickness is
typically a chronic disease, making it a difficult disease to diagnose
in the field. Low levels of trypanosomes in circulating blood make it
difficult to detect the presence of parasites in blood smears, requiring
more sophisticated means of detecting trypanosomes such as with the use
of miniature anion-exchange / centrifugation (mAEC) technique. In
comparison to the East African form, T. b. gambiense has a
longer evolutionary history with humans, having successfully adapted to
establishing infections in human hosts without manifesting severe
symptoms. Astonishingly, infection rates of T. b. gambiense in wild glossina populations are as low 0.1%, even in areas with an epidemic of sleeping sickness (Jordan, 1986).
Vectors of the West African
sleeping sickness are species of the palpalis group, most of which are
in close contact with humans. Several different reservoirs for T. b. gambiense
have been identified, strongly suggesting that the persistence of
sleeping sickness in human populations may be maintained by other
animals, such as the African domestic pig (Watson, 1962; Gibson et al.,
1978;, Mehlitz et al., 1982). However, T. b. gambiense has not
been observed or proven experimentally to reach significantly
infectious levels of parasitemia in other reservoir hosts. Although it
is widely accepted that the human-to-fly contact is the main route of
transmission, some suggest a minor cycle involving an animal reservoir
may help explain the re-emergence and persistence of the disease in West
Africa (Noireau et al,. 1989).
The epidemiology of T. b. gambiense
sleeping sickness is far from being fully understood. Despite the low
levels of parasitemia in humans, the disease has successfully
established endemicity in many regions of West Africa . It has also long
been observed that the incidence of disease is not related to the
density of the glossina populations, and that epidemics often occur in
areas where the density of the vector is low (Jordan, 1986). In Nigeria,
sleeping sickness occured in the north where the distribution of G. p. palpalis and G. tachinoides
were scarce and restricted to vegetation close to watercourses during
the dry season (Edeghere et al., 1989). In Southern Nigeria, the same
species of tsetse flies are found in abundance due to favorable climatic
conditions, yet cases of sleeping sickness have never been observed. It
is thought that the nature of the human-fly contact is of particular
importance in the transmission of T. b. gambiense and the
distribution of the disease, and that human-fly contacts can be
classified as “personal” or “impersonal” depending on the ecological
circumstances of the interaction (Nash and Page, 1953). “Personal”
contact refers to situations where fly movements are restricted to areas
where exposure to humans are frequent, such as a watering hole or a
stream, and single tsetse fly can have multiple opportunities to feed on
humans. “Impersonal” contact occurs when fly movements are less
restricted, and where repeated contacts are not likely. In general,
ecological isolation of tsetse flies in the vicinity of human
populations lead to increased “personal” contact. Climatic stress, lack
of natural hosts where humans have destroyed wild animals close to
villages, or clearing vegetation for cultivation are all examples of
restrict movements of palpalis group vectors.
5.2 |
Epidemiology of East African Sleeping Sickness |
|
East African sleeping sickness
differs from West African sleeping sickness in both its epidemiology as
well as its clinical manifestations in mammalian hosts (Baker, 1974).
The clinical symptoms of East African sleeping sickness are more severe,
and the onset of the disease is rapid. In contrast to T. b. gambiense, T. b. rhodensiense occurs with higher levels of parasitemia in ungulates, and humans are the adventitious hosts. The vectors of T. b. rhodensiense are the G. mositans subspecies, G. pallidipes and G. swnnertoni species from the morsitans group, and on lesser occasions the peridomestic vectors from the palpalis group, G. fuscipes and G. tachinoides .
Sporadic cases usually arise from among those in the population whose
activities bring them into contact with the savannah woodland habitats
of the morsitans group. Although the vectors normally feed on game
animals, under extreme situations where “personal” contact is increased
due to social and/or environmental factors, a human-fly-human
transmission cycle may ensue resulting in an outbreak. Droughts and
political turmoil are known to increase the number of cases when entire
communities relocate to hitherto unoccupied areas in search of safety or
fertile lands and water (Molyneaux and Ashford, 1983).
5.3 |
Clinical Features and Disease Onset |
|
The clinical manifestations of
both forms of sleeping sickness are usually quite different, but can be
easily confused because of the variability of symptoms and length of
time until onset depends heavily on host characteristics (Molyneaux and
Ashford, 1983). The chancre, a leathery swelling at the site of the
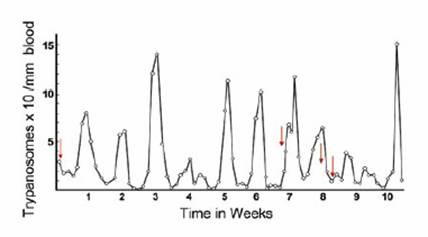
bite, is usually the first symptom of the disease, primarily for T. b. rhodensiense. Within weeks, those with opportunistic levels of infection with T. b. rhodensiense start to experience irregular intermittent fevers associated with the waves of parasitaemia that are characteristic of T. b. rhodensiense infections. For T. b. gambiense,
lymphoadenopathy occurs more frequently. Oedema of the face is another
frequent sign of infection, and anemia may be present, particularly in T. b. rhodensiense.
There are two stages to sleeping
sickness; the early stage refers to the hemolymphatic infection, and the
late stage refers to infection of the CNS. The development of late
stage sleeping sickness may not occur for decades in West African
sleeping sickness, and a patient may only suffer mildly from fatigue due
to the occasional rises of parasites in the blood. However, East
African sleeping sickness is far more virulent, and can develop into
late stage sleeping sickness within weeks. Although symptoms and signs
associated with nervous system involvement are varied for both East and
West African sleeping sickness, advanced disease epileptic attacks,
maniacal behavior, somnolence and coma are some typical late stage
symptoms (Dumas and Bisser, 1999). Both treatment options and survival
rates are drastically reduced once the trypanosomes infect the CNS.
Today, there are only a handful
of active drugs available for treatment of human African
trypanosomiasis. No significant development has been made over the last 2
decades. The current line of treatment is problematic for many reasons:
firstly, the drugs are harmfully toxic requiring extensive
hospitalization. Secondly, regular follow-ups to check for relapse is
essential but difficult in many of the areas where sleeping sickness is
endemic.
Summary of drugs available for treatment of human African trypanosomiasis
(adapted from Bouteille et al,. 2003)
| Drug |
Marketed |
Specgtrum of Activity |
Stage of disease |
Suramin
(Germanin) |
1922 |
T. b. rhodensiense |
Stage 1 |
Pentamidine
(Pentacarinat) |
1937 |
T. b. gambiense |
Stage 1 |
Melarsoprol
(Arsobal) |
1949 |
T. b. gambiense
T. b. rhodensiense
|
Stage 1 & 2
Stage 1 & 2 |
Eflormithine
(Orindyl) |
1981 |
T. b. gambiense |
Stage 1 & 2 |
Treatment of the hemolymphatic stage is based on pentamidine and suramine . Melarsoprol,
an arsenic compound, is the only treatment option available for late
stage sleeping sickness because of its ability to penetrate the
blood-brain barrier. Unfortunately, even when administered under careful
medical attention, the treatment has a mortality rate as high as 12 %
(Apted, 1957). Eflornithine is effective against both stages of T.b gambiense infection, but not against T. b rhodensiense
(Iten et al., 1995). Although the most recent and effective drug
against sleeping sickness, it is not widely available, difficult to
administer, and costly for use under African health care conditions
(Bouteille et al., 2003) So far, only 2000 patients in therapeutic
trials have been treated with eflornithine.
Use of pentamidine as a form of mass
chemoprophylaxis has proven to be an effective form of prevention and
control in endemic foci of T. b. gambiense.
6 |
Medical Ecology of Sleeping Sickness
Part III: Prevention and Control |
|
In the absence of a vaccine for
trypanosomosis and with the looming threat of further trypanocidal drug
resistance, the most theoretically desirable means of controlling the
disease is through controlling the vector population (Leak, 1999).
Although complete eradication of the vector is impossible, the most
successful attempts at controlling tsetse flies are likely to be at the
extreme limits for survival of the fly, where both the density of the
fly is low and “personal” contact with humans may be highest (Rogers,
1979).
 |
| |
There are several different
control techniques available today, but the use of chemicals in
controlling tsetse populations is still the most common method. In
brief, whether aerial or from the ground, residual insecticides such as
organochlorines (DDT, Dieldrin, Endosulfan), pyrethroids (deltamethrin,
permethrin, and alphamethrin), and avermectins (ivermectin) are used to
target areas where human-to-fly contact are likely. Pyrethroids are
preferred because they are rapidly degraded in soil and are
environmentally safe, unlike organochlorines, carbamates and
organophosphates that bioaccumulate in the food chain and are highly
toxic to mammals and other vertebrates. Despite being effective, the use
of organochlorines and organophosphates are now banned for widespread
outdoor spraying. Susceptibility to insecticides varies from one species
to another, and between the different classes of species (Leak, 1999).
The most common form of administering insecticides is through the use of
pressurized knapsacks. Over 200,000 km2 of tsetse-infested
land has been cleared by ground-spraying in West Africa, mainly in
Nigeria, and proved to be successful and cost-effective (Barrett, 1997).
Although the process is highly labor intensive and limited in
geographical scope, the spraying is administered discriminatively to day
and night resting sites during the dry season and are much more
effective than indiscriminate spraying from the air or from vehicles.
 |
| |
Traps and targets are mechanical
devices used to kill or weaken tsetse flies through insecticides or
various trapping methods. The use of traps and targets to control tsetse
populations have been successful primarily because tsetse flies are
k-strategists with a low rate of reproduction, and require very little
sustained mortality pressure to bring about a reduction in population or
even eradication from an area (Weidhaas and Haile, 1978). Haargrove
(1988) estimated that an additional mortality of 4% per day imposed on
female flies was enough to cause extinction, in the absence of
immigration. The traps and targets attract tsetse flies by taking
advantage of their primary host-seeking behaviors, visual and olfactory
stimulation. The developments of potent attractants in the last 20 years
as well as the production of second-generation synthetic pyrethroid
insecticides are making this form of control technique highly successful
(Wall and Langely, 1991).
There are many prototypes of
traps and targets customized to attract as many tsetse flies as possible
in different environments, with a strong emphasis on designs that are
easy to duplicate and maintain locally. Although most traps are strongly
reliant on chemical attractants and insecticides, some have recently
been designed to attract tsetse flies based on visual stimulation alone
and to kill tsetse flies through a trapping mechanism (NGU and NG2B
traps). Although these traps may not be as efficient in attracting and
killing tsetse flies, they are far more affordable and feasible to
implement in resource poor settings. Such traps were used to
successfully suppress the tsetse fly populations in Nguruman, Kenya .
With the Maasai community involved, 190 homemade NG2B traps were
deployed over 100 km2, and a 98-99% G. pallidipes reduction was achieved over a 10 month period, and the reinvasion was kept relatively low during the rainy season (Dransfield et al,
1990). Targets and traps are usually deployed in and around areas where
human-fly contacts are greatest, such as streams frequented by
villagers, or fringes of cultivated fields. All aspects of these targets
and traps, from its design and color to their strategic placement, are
reliant on understanding the biology and behavioral ecology of the
various tsetse fly species.
Exploiting the knowledge that
tsetse flies concentrated in certain areas lead to numerous
bush-clearing projects all over West and East Africa to drastically
alter and maintain the area unsuitable for tsetse fly habitation.
Discriminative bush-clearing was used in Uganda to control for G.m. centralis
by clearing taller Acacia trees in the Ankole district (Harley and
Pilson, 1961). In Tanzania, between 1923 and 1930, bush-clearing methods
were also widely employed to stop the spread of sleeping sickness
epidemic in Maswa district, where G. swynnertoni was prevalent
(Leak, 1999). Similar tactics were used in Ghana to control sleeping
sickness around villages were human-fly contacts were high (Morris,
1949). Despite the apparent success of these methods, it is widely
accepted that bush-clearing is unsuitable as a long term control measure
due to the expense and speed of reinvasion, as well as the
environmental damage it causes through soil erosion, decreased soil
fertility, and its adverse effects on water supplies.
6.4 |
Sterile Insect Technique |
|
One of the more modern methods of
non-insecticidal control is the Sterile Insect Technique (SIT) which
was first considered as a means to control tsetse by Simpson in 1958.
This technique relies on the mating of wild females with sterile male
flies. Physiologically, female tsetse flies are only required to mate
once to store sperm in its spermathecae in sufficient quantity such that
fertilization can occur over its entire reproductive life. Mating with a
sterile male would thus result in no offspring. However, SIT was
considered to be impractical for control of high-density tsetse
populations above 1000 males per square mile due to the large number of
sterilized males that would be required. For SIT to be effective, it has
been estimated that 10% of the females in the population need to be
inseminated, and in order to achieve that, the number of sterile males
released must constitute 80% of the male population (Rogers and
Randolph, 1985).
Sterilization of male tsetse flies can be carried out by
Irradiation
Gamma rays, Beta rays
Chemosterilization
Bisazir, Metepa, Tepa, Apholates, Phytosterols
Physiological sterilization
Pyriproxyfen, Sulphaqunizaline, Chlordimeform
In 1994, an eradication program
conducted in Zanzibar by the authorities and the International Atomic
Energy Agency (IAEA) used a combination of insecticide-impregnated traps
and SIT to completely eradicate the entire tsetse population of
Zanzibar by 1996 (IAEA, 1997). Over 7.8 million gamma irradiated sterile
male G. austeni flies were released over the island with a
ratio of 50:1 sterilized males to wild males. This campaign may have
been successful in part because there is virtually no immigration of
tsetse flies into the island.
6.5 |
The Future of Sleeping Sickness Control |
|
After the publication of works
such as “Silent Spring” (Carson, 1962), public awareness of the dangers
associated with insecticides are increasingly changing the way we treat
our environment, and the way we institute environmental controls.
Consequently, efforts to introduce more environmentally friendly methods
of vector control, such as the use of traps without insecticides,
challenges us to understand more about the vectors that transmit the
disease, as well as the ecological balance that we - as humans - strike
with them.
We live in a world where various
technical means of control are available to address the spread of the
disease. However, sleeping sickness is a disease of the developing
world, where despite the multitude of control strategies, the issues
have widely been neglected and abandoned. One of the key components
required to bring about effective change is to consider the
sustainability of the control strategy, and to encourage local
communities to take ownership over the process, thereby empowering
people to take an active role in an environmentally conscious solution.
Increasing knowledge through culturally sensitive education, providing
technical support, and a long-term commitment of basic resources to
beneficiary communities is essential for large-scale tsetse control
(Swynnerton, 1925).
Alongside efforts to reduce the
spread of disease through environmental controls, there is also an
urgent need to improve current surveillance and diagnostic procedures.
Mortality can be drastically reduced when cases can be diagnosed early
enough to prevent the progression of late-stage sleeping sickness.
Training and resources are desperately needed in endemic areas for
proper diagnostics and sero-surveillance.
 |
| |
Perhaps the most mysterious
aspect of this disease relates to the issue of treatment options, and
the availability of drugs in Africa . Drug and vaccine development for
diseases in developing countries have always been lagging, and
unfortunately, trypanocidal drugs are no exception. An estimated 300,000
– 500,000 people are currently infected and suffering from the disease
with no hope for treatment. In 2000, the USFDA approved the use of
eflornithine by the Bristol-Myers Squibb Company and The Gillette
Company in a product called Vaniqa TM,
a topical eflornithine HCl cream to remove facial hair. Perhaps some of
the profits generated from the sale of this form of the drug will be
used to underwrite the free use of the drug in Africa, similar to what
has already happened at Merck, who donates invermectin for the treatment
of river blindness, and at Pfizer Inc for their azithormycin give away
program for the treatment of trachoma.
Atkins J. (1978) Sleeping Sickness. Tropical Medicine and Parasitology, Classic Investigations. Cornell University Press, Ithaca, New York, 181-182
Baker J.R. (1974) Epidemiology of African sleeping sickness. Trypanosomiasis and Leishmaniasis with Special Reference to Chagas Disease. CIBA Foundation Symposium, 29-50.
Baldry D.A.T. (1980) Loca
distribution and ecology of Glossina palpalis and G. tachinoides in
forest foci of west African human trypanosomiasis, with special
reference to associations between peri-domestic tsetse and their hosts. Insect Science and its Application, 1, 85-93.
Barret J.C. (1997) Control strategies for African trypanosomiases: their sustainability and effectiveness. Trypanosomiasis and Leishmaniasis. CAB International, Wallingford, 347-362.
Bouteille B. et al. (2003) Treatment perspective for human African trypanosomiasis. Fundamental & Clinical Pharmacology, 17, 171-181.
Brady J. (1972) The visual
responsiveness of the tsetse fly Glossina morsitans Westwood
(Glossinidae) to moving objects: the effects of hunger, sex, host odor
and stimulus characteristics. Bulletin of Entomological Research, 62, 257-279.
Brady J., Crump A.J. (1978) The control of circadian activity rhythms in tsetse flies: environment or physiological clock? Physiological Entomology, 3, 177-190.
Cattand P. (1994) World Health Organization Press Release. WHO/73, 7 October 1994.
Dransfield R.D. et al. (1990)
Control of tsetse fly (DipteraL Glossinidae) populations using traps at
Nguruman, south-west Kenya . Bulletin of Entomological Research, 80, 265-276.
Dumas M., Bisser S. (1999) Clinical aspects of human African trypanosomiasis (Ch. 13), Progress in human African rypanosomiasis, sleeping sickness . Springer-Verlag, Paris, 215-233.
Edeghere H. et al. (1989) Human African trypanosomiasis (sleeping sickness): new endemic foci in Bendel state, Nigeria . Tropical Medicine and Parasitology, 40, 16-20.
Ford J. (1960) The influence of tsetse flies on the distribution of African cattle. Proceedings of the 1 st Federal Scientific Congress, Salisbury, 357-365
Gibson W.C. et al. (1978) The
identification of Trypanosoma brucei gambiense in Liberian pigs and dogs
by isoenzymes and by resistance to human plasma. Tropenmedizin und Parasitologie, 29, 335-345.
Glasgow J.P. (1963) The distribution and abundance of tsetse. International Series of Monographs on Pure and Applied Bioology, 20, Pergamon Press, Oxford, 241.
Hargrove J.W. (1981) Tsetse: the limits to population growth. Medical and Veterinary Entomology, 2, 203-217.
Harley J.M.B., Pilson R.D. (1961)
An experiment in the use of discriminative clearing for the control of
Glossina morsitans westwood in Ankole, Uganda . Bulletin of Entomological Research, 52, 561-576.
Hendrickx G., Napala, A. (1997)
Le contròle de la trypanosome ‘á la carte': une approche intègrèe basèe
sur un Système d'Information Gèographique. Acadèmie Royale des Sciences d'Outhre Mer. Mèmoires Classe Sciences Naturelles and Mèdicales. In 8 th Nouvelle Sèrie, Tome24-2, Bruxelles, 1997, 100.
Jackson C.H.N. (1950) Pairing of Glossina morsitans Westwood with G. swynnertoni Austen (Diptera). Proceedings of the Royal Entomological Society of London (A), 25, 106.
Jaenson T.G.T. (1980) Mating behavior of females of Glossina pallidipes. Bulletin of Entomological Research, 70, 49-60.
Jahnke H.E. (1982) Livestock Production Systems in Livestock Development in Tropical Africa . Kieler Wissenschaftsverlag Vauk, Kiel, FRG.
Jordan A.M. (1974) Recent
developments in the ecology and methods of control of tsetse flies
(Glossina spp.) (Diptera, Glossinidae) – a review. Bulletin of Entomological Research, 63, 361-399.
Jordan A.M. (1976) Tsetse flies as vectors of trypanosomes. Veterinary Parsitology, 2, 143-152.
Jordan A.M. (1986) Trypanosomiasis Control and African Rural Development. Longman, London .
Kazadi J.M.L. et al., (1991)
Etude de la capacitè vectorielle de Glossina palpalis gambiensis
(Bobo-Dioulasso) vis-á-vis de Trypanosoma brucei brucei EATRO 1125. Revue d'Élevage et de Mèdecine Vètèrinaire des Pays Tropicaux, 44, 437-442.
Knudsen A.B., Slooff R. (1992) Vector-borne disease problems in rapid urbanization: New approaches to vector control. Bulletin of the World Health Organization, 70, 1-6.
Kuzoe F.A.S et al. (1985)
Observations on an apparent population extension of Glossina tachinoides
Westwood in southern Ivory Coast . Insect Science and its Application, 6, 55-58.
Leak S.G.A. (1999) Tsetse Biology and Ecology, CABI publishing, New York .
Le Ray D. (1989) Vector susceptibility to African trypanosomes. Annales de la Société Belge de Médecine Tropicale, 69, Supplément 1, 167-171
Maudlin I. et al. (1990) The
relationship between Rickettsia-like organisms and trypanosome
infections in natural populations of tsetse in Liberia . Tropical Medicine and Parasitology, 421, 265-267.
McKelvey J. J. (1973) Man Against Tsetse: Struggle for Africa . Cornell University Press, Ithaca and London .
Mehlitz D. et al. (1982)
Epidemiological studies on the animal reservoir of Gambiense sleeping
sickness. Part III. Characterization of Trypanozoon stocks by isoenzymes
and sensitivity to human serum. Tropenmedizin und Parasitologie, 33, 113-118.
Mihok S. et al. Trypanosome
infection rates in tsetse flies (Diptera: Glossinidae) and cattle during
control operations in the Kagera river in Rwanda . Bulletin of Entomological Research. 82, 361-367.
Moloo S.K. (1993) The distribution of Glossina species in Africa nd their natural hosts. Insect Science and its Application, 14, 511-527.
Molyneaux D.H. (1980) Host-trypanosome interactions in Glossina. Insect Science and its Application, 1, 39-46.
Molyneux D.H., Ashford R.W. (1983) The Biology of Trypanosoma and Leishmania, Parasite of Man and Domestic Animals. Taylor and Francis, London .
Morris K.R.S. (1949) Planning the control of sleeping sickness. Transactions of the Royal Society of Tropical Medicine and Hygiene, 43, 165-198.
Nash T.A.M., Page W.A. (1953) On the ecology of Glossina palpalis in northern Nigeria . Transactions of the Royal Entomological Society of London, 104, 71-170.
Noireau F. et al. (1989) The epidemiological importance of the animal reservoir of Trypanosoma brucei gambiense in the Congo . Tropical Medicine and Parasitology, 40, 9-11.
Rogers D.J. (1979) Tsetse population dynamics and distribution. A new analytical approach. Journal of Animal Ecology, 48, 825-849.
Rogers D.J., Randolph S.E. (1985) Population ecology of tsetse. Annual Review of Entomology, 30, 197-216.
Swynnerton C.F.M. (1925) The tsetse fly problem in the Nzega sub-district Tanganyika Territory . Bulletin of Entomological Research, 16, 99-110.
Teesdale C. (1940) Fertilization in the tsetse fly, Glossina palpalis, in a population of low density. Journal of Animal Ecology, 9, 24-26.
Wall R., Langley P.A. (1991) From
behavior to control: The development of traps and target techniques for
tsetse fly population management. Agricultural Zoology Reviews, 4, 137-159.
Watson H.J.C. (1962) The domestic pig as a reservoir of Trypanosoma gambiense. ISCTR 9 th meeting, Conakry, 327.
Weidhaas D.E., Haile D.G. (1978) The domestic pig as a reservoir of Trypanosoma gambiense. ISTR 9 th meetings. Conakry, 327.
WHO (1995) Planning Overview of Tropical Diseases Control. Division of Tropical Diseases. World Health Organization, Geneva .
Williamse L.P.M, Takken W. (1994) Odor-inducedhost location in tsetse flies (Diptera: Glossinidae). Journal of Medical Entomology, 31, 774-794.
TRYPANOSOMIASIS - SOUTH AFRICA EX MALAWI (KASUNGU NATIONAL PARK)
A ProMED-mail post
Date: Sat, 24 Dec 2005
From: Lucille Blumberg <lucilleb@nicd.ac.za>
2 cases of East African trypanosomiasis were
confirmed in Johannesburg, South Africa in the last month. Both
patients most likely acquired the disease in the Kasungu National Park,
Malawi. The 1st was a British soldier who took part in a
field exercise in Malawi. The 2nd was a South African tourist who was
on a trans-Africa overland safari. Both patients noticed large, single
erythematous skin lesions (in the groin and on the foot,
respectively) while in the Kasungu National Park and reported numerous
tsetse fly bites. Both patients visited the park within a 2-week
period in the middle of November 2005. The British soldier presented a
week after the tsetse fly bite with fever and multi-organ failure. He
had renal failure, thrombocytopenia, raised liver enzymes, and
evidence of possible cardiac involvement with a bradycardia and
features suggestive of Wenkebach-type [atrioventricular] heart block.
The 2nd patient presented about 10 days after being bitten with fever
and drowsiness and was investigated as possible malaria.
In both cases the diagnosis was confirmed in the
laboratory on blood smear examination. Both patients responded
well to suramin. The South African tourist, although clinically well,
is still being followed up to exclude central nervous system
involvement.
Dr Lucille Blumberg and Prof John Frean,
Epidemiology and Parasitology Reference Units National Institute for
Communicable Diseases Johannesburg, South Africa NICD, No 1
Modderfontein Road, Sandringham, Johannesburg <lucilleb@nicd.ac.za>
Trypanosomiasis has been emerging in Central
Africa over the past decade, but this is the 1st time that ProMED has
had a report of cases which definitely were infected in Malawi. There
are 8 human trypanosomiasis-affected districts out of the 25 districts
in Malawi: Rumphi in the northern region; Kasungu, Ntchisi, and
Nhotakota in the central region; and Mangochi, Machinga, Chikwawa, and
Mulanje in the southern region. The awareness level regarding human
trypanosomiasis is very low among both health workers and the
community (source: <http://www.fao.org/paat/html/mwi.htm>).
The local reactions at the site of tsetse fly bites are characteristic of Trypanosoma brucei rhodesiense and are associated
with regional lymphadenopathy. Early treatment
is life-saving, and trypanosomiasis should be considered in
differential diagnosis in
travellers from Malawi. Data on indigenous cases will be greatly appreciated. - Mod.EP]
see also:
Trypanosomiasis - Uganda (Kaberamaido)(02): background 20050829.2552
Trypanosomiasis - USA ex Tanzania (Serengeti): RFI 20050713.1989
2001
Trypanosomiasis - Europe ex Tanzania (05) 20010624.1197
Trypanosomiasis - Europe ex Tanzania (04) 20010618.1169
Trypanosomiasis - Kenya 20010511.0912
Trypanosomiasis - Uganda (02) 20010421.0781
2000
Trypanosomiasis, African - Australia ex Tanzania 20001107.1943
Trypanosomiasis, African: emerging (02) 20000816.1363
Trypanosomiasis, African: emerging 20000808.1320
1998
Trypanosomiasis - Uganda 19980508.0899]
[Back To Top] |
|